Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2020-10-25 , DOI: 10.1016/j.snb.2020.129084 Arul Pundi , Chi-Jung Chang , Jemkun Chen , Shih-Rong Hsieh , Ming-Ching Lee
|
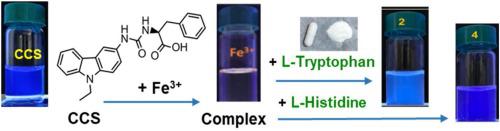
Quantitative analysis of the L-tryptophan content in commercial products by chemosensor has not been reported yet. In this study, the chiral carbazole based sensor (CCS), bearing a chiral “urea-carboxylic acid” binding site was prepared. CCS was proved to be effective in the consecutive detection of Fe3+, L-tryptophan (Trp), and L-histidine (His) by naked eyes. CCS exhibits different color changes and fluorescent ‘turn-off’ response toward Fe3+ and Fe2+. The discrimination of these two oxidation states of iron by CCS is important to understand the iron-related biological reactions. The limit of detection for Fe3+ by CCS is 84 nM in a high water-content solution (99 % water). CCS sensor can act as a reversible fluorescence quenching sensor for Fe3+. The results of Job’s plot analysis and mass spectra reveal that Fe3+ binds to CCS with a 1:1 stoichiometry. The CCS-Fe3+ complex can sense tryptophan, or histidine by a dose-dependent turn-on fluorescent response in a high water-content solution (99 % water). The mass spectra suggest the sensing of L-histidine or L-tryptophan by CCS-Fe3+ was performed with a CCS-Fe3+: amino acid binding ratio of 1:1. In comparison with other chemosensors reported in the literature, CCS can detect the L-Trp and L-His with a low limit of detection (0.31 μM L-Trp, 7.64 μM L-His) in a high water content environment. Furthermore, the CCS - Fe3+ complex based chemosensor was successfully applied to quantify the L-tryptophan content in commercial sleep-improving capsules containing various ingredients. The measured L-tryptophan contents in two samples are close to the announced values.
中文翻译:

基于手性咔唑的传感器,用于连续“开-关-开”荧光检测Fe 3+和色氨酸/组氨酸
尚未有化学传感器对商业产品中L-色氨酸含量进行定量分析的报道。在这项研究中,制备了带有手性“脲-羧酸”结合位点的手性咔唑基传感器(CCS)。事实证明,CCS可以通过肉眼连续检测Fe 3+,L-色氨酸(Trp)和L-组氨酸(His)。CCS对Fe 3+和Fe 2+表现出不同的颜色变化和荧光“关闭”响应。CCS对铁的这两种氧化态的区分对于理解铁相关的生物反应非常重要。Fe 3+的检出限在高水含量的溶液(99%的水)中,CCS的分析值为84 nM。CCS传感器可作为Fe 3+的可逆荧光猝灭传感器。乔布的图分析和质谱结果表明,Fe 3+以1:1的化学计量比结合CCS。CCS-Fe 3+复合物可以在高水含量溶液(99%的水)中通过剂量依赖性的开启荧光响应来检测色氨酸或组氨酸。质谱表明L-组氨酸或L-色氨酸通过CCS-Fe的感测3+用CCS-铁进行3+:1氨基酸结合:1的比例。与文献中报道的其他化学传感器相比,CCS可以以低检测限检测L-Trp和L-His (在高含水量环境中为0.31μML-Trp,7.64μML-His)。此外,基于CCS-Fe 3+络合物的化学传感器已成功应用于量化含有各种成分的商业睡眠改善胶囊中L-色氨酸的含量。在两个样品中测得的L-色氨酸含量接近公布的值。































 京公网安备 11010802027423号
京公网安备 11010802027423号