Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-10-21 , DOI: 10.1016/j.jcis.2020.10.059 Wanjia Zhang , Zhitao Feng , Yuhang Yang , Wei Sun , Stephen Pooley , Jian Cao , Zhiyong Gao
|
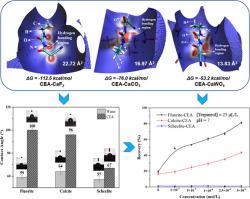
Mono-functional chelating collectors exhibit limited selectivity in the flotation of minerals. In particular, the selective separation of calcium minerals presents a significant challenge because mono-functional chelating collectors, such as fatty acid, indistinguishably adsorb onto mineral surfaces by coordinating with the same metal cation (Ca2+). Thus, there is an urgent need to develop new-mode-functional collectors to separate calcium minerals and a need to understand the underlying chemoselectivity. Given the difference of the hydrogen bonding ability of anions with fluorite, calcite and scheelite surfaces, the introduction of additional hydrogen bonding functional groups into collector molecules is a novel strategy to improve selectivity. In this study, a hydrogen and coordination bonding (bi-functional) collector, 2-cyano-N-ethylcarbamoyl acetamide (CEA) was developed, which could form coordination bonds with the Ca2+ ions (by carbonyl groups) and hydrogen bonds with the anions (by amino groups) on calcium mineral surfaces. The results of flotation tests showed that CEA can selectively separate fluorite and calcite from scheelite at pH 7. The promising selectivity of CEA lies in both the electrical properties and the anions’ hydrogen bonding ability with the three calcium minerals. The negatively charged scheelite surfaces are not conducive to coordination bonding with CEA while the positively charged fluorite and calcite surfaces are. Besides, the hydrogen bonding ability of fluorite (F−) and calcite (CO32−) with carbamido in CEA is higher than that of scheelite (WO42−), and this also plays an essential role. This coordination and hydrogen bonding based surfactant design protocol has a great potential in the development of tail-made collectors/depressants for the separation of other oxidized minerals.
中文翻译:

双功能氢和配位键表面活性剂:新型有前途的捕收剂,可改善钙矿物质的分离
单功能螯合捕集剂在矿物浮选中显示出有限的选择性。特别是,钙矿物质的选择性分离提出了重大挑战,因为单功能螯合捕集剂(例如脂肪酸)通过与相同的金属阳离子(Ca 2+)。因此,迫切需要开发新型功能的捕收剂来分离钙矿物质,并且需要了解潜在的化学选择性。考虑到阴离子与萤石,方解石和白钨矿表面的氢键合能力不同,将额外的氢键合官能团引入捕收剂分子是提高选择性的新策略。在这项研究中,开发了氢和配位键(双功能)捕收剂2-氰基-N-乙基氨基甲酰基乙酰胺(CEA),它可以与Ca 2+形成配位键。离子(通过羰基)和氢离子(通过氨基)与钙矿物表面上的阴离子键合。浮选试验的结果表明,CEA可以在pH 7时从白钨矿中选择性地分离萤石和方解石。CEA的有希望的选择性在于电学性质和阴离子与三种钙矿物质的氢键结合能力。带负电的白钨矿表面不利于与CEA的配位键合,而带正电的萤石和方解石表面则不利。此外,萤石的氢键能力(F - )和方解石(CO 3 2-与CEA脲)比白钨(WO更高4 2-),这也起着至关重要的作用。这种基于配位和氢键的表面活性剂设计方案在开发用于分离其他氧化矿物的尾捕集剂/抑制剂方面具有巨大的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号