Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-10-29 , DOI: 10.1016/j.jcat.2020.10.018
Huong Lan Huynh , Jie Zhu , Guanghui Zhang , Yongli Shen , Wakshum Mekonnen Tucho , Yi Ding , Zhixin Yu
|
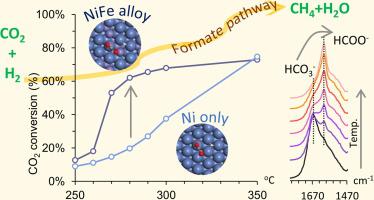
Bimetallic NiFe catalysts have emerged as a promising alternative to the traditional Ni catalysts for CO2 methanation. However, the promoting effect of Fe on the bimetallic catalysts remains ambiguous. In this study, a series of NiFe catalysts derived from hydrotalcite precursors were investigated. In situ x-ray diffraction (XRD) analysis revealed that small NiFe alloy particles were formed and remained stable during reaction. When Fe/Ni = 0.25, the alloy catalysts exhibited the highest CO2 conversion, CH4 selectivity and stability in CO2 methanation at low temperature of 250–350 °C. The in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) study indicated that the formate pathway was the most plausible reaction scheme on both Ni and NiFe alloy catalysts, while a moderate addition of Fe facilitated the activation of CO2 via hydrogenation to *HCOO. Density functional theory (DFT) calculations further demonstrated that the overall energy barrier for CH4 formation was lower on the alloy surface.
中文翻译:

原位DRIFTS和DFT研究Fe对CO 2甲烷化负载Ni催化剂的促进作用。
双金属NiFe催化剂已成为有前途的CO 2甲烷化甲烷Ni催化剂的替代品。但是,Fe对双金属催化剂的促进作用仍然不清楚。在这项研究中,研究了一系列由水滑石前体衍生的NiFe催化剂。原位X射线衍射(XRD)分析表明,形成了小的NiFe合金颗粒,并在反应过程中保持稳定。当Fe / Ni = 0.25时,合金催化剂在250–350°C的低温下表现出最高的CO 2转化率,CH 4选择性和CO 2甲烷化的稳定性。在原位漫反射红外傅里叶变换光谱法(DRIFTS)的研究表明,甲酸途径是在Ni和NiFe合金催化剂上最合理的反应方案,而适度添加的Fe则通过加氢生成* HCOO促进了CO 2的活化。密度泛函理论(DFT)计算进一步证明,在合金表面形成CH 4的总能垒较低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号