iScience ( IF 4.6 ) Pub Date : 2020-10-21 , DOI: 10.1016/j.isci.2020.101711
Qing Wei 1 , Audra A Hargett 2 , Barbora Knoppova 1 , Alexandra Duverger 3 , Reda Rawi 4 , Chen-Hsiang Shen 4 , S Katie Farney 4 , Stacy Hall 1 , Rhubell Brown 1 , Brandon F Keele 5 , Sonya L Heath 3 , Michael S Saag 3 , Olaf Kutsch 3 , Gwo-Yu Chuang 4 , Peter D Kwong 4 , Zina Moldoveanu 1 , Milan Raska 1, 6 , Matthew B Renfrow 2 , Jan Novak 1
|
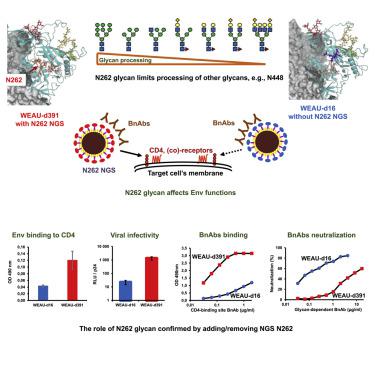
HIV-1 envelope (Env) N-glycosylation impact virus-cell entry and immune evasion. How each glycan interacts to shape the Env-protein-sugar complex and affects Env function is not well understood. Here, analysis of two Env variants from the same donor, with differing functional characteristics and N-glycosylation-site composition, revealed that changes to key N-glycosylation sites affected the Env structure at distant locations and had a ripple effect on Env-wide glycan processing, virus infectivity, antibody recognition, and virus neutralization. Specifically, the N262 glycan, although not in the CD4-binding site, modulated Env binding to the CD4 receptor, affected Env recognition by several glycan-dependent neutralizing antibodies, and altered site-specific glycosylation heterogeneity, with, for example, N448 displaying limited glycan processing. Molecular-dynamic simulations visualized differences in glycan density and how specific oligosaccharide positions can move to compensate for a glycan loss. This study demonstrates how changes in individual glycans can alter molecular dynamics, processing, and function of the Env-glycan shield.
中文翻译:

聚糖定位影响 HIV-1 Env 聚糖屏蔽密度、功能和抗体识别
HIV-1 包膜 (Env) N-糖基化影响病毒细胞进入和免疫逃逸。目前尚不清楚每种聚糖如何相互作用形成 Env-蛋白-糖复合物并影响 Env 功能。在这里,对来自同一供体的两种 Env 变体的分析,具有不同的功能特征和 N-糖基化位点组成,表明关键 N-糖基化位点的变化会影响远处的 Env 结构,并对 Env 范围的聚糖加工、病毒感染性、抗体识别和病毒中和产生连锁反应。具体来说,N262 聚糖虽然不在 CD4 结合位点,但调节了 Env 与 CD4 受体的结合,影响了几种聚糖依赖性中和抗体对 Env 的识别,并改变了位点特异性糖基化异质性,例如,N448 显示出有限的聚糖加工。分子动力学模拟可视化了聚糖密度的差异以及特定寡糖位置如何移动以补偿聚糖损失。本研究展示了单个糖基的变化如何改变 Env-糖基屏蔽层的分子动力学、加工和功能。





































 京公网安备 11010802027423号
京公网安备 11010802027423号