当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid-liquid equilibrium for n-hexane + benzene + sulfolane, + 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][NTf2]), + 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]) and + the mixtures of [EMIM][NTf2] and [EMIM][EtSO4]
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.fluid.2020.112882 Yicang Guo , Fengming Shi , Qipeng Shu , Xiaoyong Yue , Cheng Wang , Lei Tao , Jinlong Li
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.fluid.2020.112882 Yicang Guo , Fengming Shi , Qipeng Shu , Xiaoyong Yue , Cheng Wang , Lei Tao , Jinlong Li
|
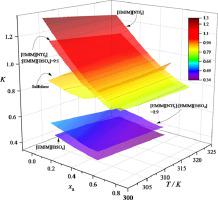
Abstract The liquid-liquid equilibrium (LLE) data for three ternary mixtures of n-hexane + benzene + sulfolane, + 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][NTf2]) and + 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]) and two quaternary ones of n-hexane + benzene + mixed ionic liquids respectively with fixed molar ratio 9:1 and 1:9 of [EMIM][NTf2] to [EMIM][EtSO4] were determined experimentally at (303.15, 313.15 and 323.15) K and atmospheric pressure. The experimental results showed that the ionic liquid of [EMIM][NTf2] captures higher molar distribution coefficient while one of [EMIM][EtSO4] is characterized with higher molar selectivity, compared to the sulfolane solvent that is extensively used for aromatic extraction in industry. The improvement of the composite separation performance for n-hexane and benzene was observed through mixing the above two ionic liquids. To represent the experimental diagram, the activity coefficient model of Non-Random Two-Liquid (NRTL) was employed to correlate the experimental data and good agreements between theoretical and experimental results were obtained. In general, the selected ionic liquid and its mixture in this work could be considered as a potential substitute for sulfolane.
中文翻译:

正己烷 + 苯 + 环丁砜、+ 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺([EMIM][NTf2])、+ 1-乙基-3-甲基咪唑鎓乙基硫酸盐([EMIM][EtSO4)的液-液平衡]) 和 + [EMIM][NTf2] 和 [EMIM][EtSO4] 的混合物
摘要 正己烷 + 苯 + 环丁砜、+ 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰)亚胺 ([EMIM][NTf2]) 和 + 1-乙基-的三种三元混合物的液-液平衡 (LLE) 数据3-甲基咪唑乙基硫酸盐 ([EMIM][EtSO4]) 和正己烷 + 苯 + 混合离子液体的两种季铵盐,[EMIM][NTf2] 与 [EMIM][ 的固定摩尔比分别为 9:1 和 1:9 EtSO4] 在 (303.15、313.15 和 323.15) K 和大气压下通过实验确定。实验结果表明,与工业上广泛用于芳烃萃取的环丁砜溶剂相比,[EMIM][NTf2]的离子液体具有更高的摩尔分布系数,而[EMIM][EtSO4]之一具有更高的摩尔选择性. 通过混合上述两种离子液体,观察到正己烷和苯的复合分离性能的提高。为了表示实验图,采用非随机两液体(NRTL)的活度系数模型来关联实验数据,并获得了理论和实验结果之间的良好一致性。一般而言,本工作中选定的离子液体及其混合物可被视为环丁砜的潜在替代品。
更新日期:2021-02-01
中文翻译:

正己烷 + 苯 + 环丁砜、+ 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺([EMIM][NTf2])、+ 1-乙基-3-甲基咪唑鎓乙基硫酸盐([EMIM][EtSO4)的液-液平衡]) 和 + [EMIM][NTf2] 和 [EMIM][EtSO4] 的混合物
摘要 正己烷 + 苯 + 环丁砜、+ 1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰)亚胺 ([EMIM][NTf2]) 和 + 1-乙基-的三种三元混合物的液-液平衡 (LLE) 数据3-甲基咪唑乙基硫酸盐 ([EMIM][EtSO4]) 和正己烷 + 苯 + 混合离子液体的两种季铵盐,[EMIM][NTf2] 与 [EMIM][ 的固定摩尔比分别为 9:1 和 1:9 EtSO4] 在 (303.15、313.15 和 323.15) K 和大气压下通过实验确定。实验结果表明,与工业上广泛用于芳烃萃取的环丁砜溶剂相比,[EMIM][NTf2]的离子液体具有更高的摩尔分布系数,而[EMIM][EtSO4]之一具有更高的摩尔选择性. 通过混合上述两种离子液体,观察到正己烷和苯的复合分离性能的提高。为了表示实验图,采用非随机两液体(NRTL)的活度系数模型来关联实验数据,并获得了理论和实验结果之间的良好一致性。一般而言,本工作中选定的离子液体及其混合物可被视为环丁砜的潜在替代品。





























 京公网安备 11010802027423号
京公网安备 11010802027423号