当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Computational Study of CO Oxidation on IrO2 (110) Surface
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148244 Chen-Hao Yeh , Bing-Cheng Ji , Santhanamoorthi Nachimuthu , Jyh-Chiang Jiang
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148244 Chen-Hao Yeh , Bing-Cheng Ji , Santhanamoorthi Nachimuthu , Jyh-Chiang Jiang
|
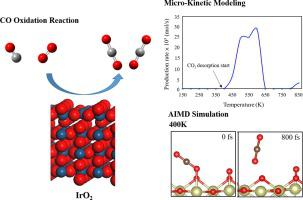
Abstract Though, oxygen-rich iridium oxide (O-IrO2 (110)) surface exhibits excellent catalytic activity towards methane activation and oxygen evolution reactions, the CO oxidation mechanism on this surface remains elusive. Here, we used density functional theory calculations, ab initio molecular dynamics (AIMD), and microkinetic simulations to explore the CO oxidation reactions via Langmuir-Hinshelwood (L-H), Eley-Rideal (E-R), and Mars-Van Krevelen (MvK) mechanisms on O-IrO2 (110) surface. We find that the C-O coupling and CO2 desorption are the rate-determining steps in L-H and E-R mechanisms, respectively. The CO oxidation via the MvK mechanism on the IrO2 (110) surface is more difficult to proceed. Both AIMD and microkinetic simulation results demonstrate that CO2 can desorb from the O-IrO2 (110) surface at 400 K. The production temperature of CO2 on the O-rich IrO2 (110) surface via E-R mechanism is lower than that via the L-H mechanism. Therefore, we predict that the O-rich IrO2 (110) surface can accelerate the CO oxidation reaction rate.
中文翻译:

IrO2 (110) 表面 CO 氧化的计算研究
摘要 尽管富氧氧化铱 (O-IrO2 (110)) 表面对甲烷活化和析氧反应表现出优异的催化活性,但该表面上的 CO 氧化机制仍然难以捉摸。在这里,我们使用密度泛函理论计算、从头分子动力学 (AIMD) 和微动力学模拟来探索通过 Langmuir-Hinshelwood (LH)、Eley-Rideal (ER) 和 Mars-Van Krevelen (MvK) 机制的 CO 氧化反应O-IrO2 (110) 表面。我们发现 CO 耦合和 CO2 解吸分别是 LH 和 ER 机制中的速率决定步骤。在 IrO2 (110) 表面上通过 MvK 机制的 CO 氧化更难进行。AIMD 和微动力学模拟结果都表明 CO2 可以在 400 K 下从 O-IrO2 (110) 表面解吸。通过ER机制在富含O的IrO2(110)表面产生CO2的温度低于通过LH机制的CO2。因此,我们预测富含 O 的 IrO2 (110) 表面可以加速 CO 氧化反应速率。
更新日期:2021-02-01
中文翻译:

IrO2 (110) 表面 CO 氧化的计算研究
摘要 尽管富氧氧化铱 (O-IrO2 (110)) 表面对甲烷活化和析氧反应表现出优异的催化活性,但该表面上的 CO 氧化机制仍然难以捉摸。在这里,我们使用密度泛函理论计算、从头分子动力学 (AIMD) 和微动力学模拟来探索通过 Langmuir-Hinshelwood (LH)、Eley-Rideal (ER) 和 Mars-Van Krevelen (MvK) 机制的 CO 氧化反应O-IrO2 (110) 表面。我们发现 CO 耦合和 CO2 解吸分别是 LH 和 ER 机制中的速率决定步骤。在 IrO2 (110) 表面上通过 MvK 机制的 CO 氧化更难进行。AIMD 和微动力学模拟结果都表明 CO2 可以在 400 K 下从 O-IrO2 (110) 表面解吸。通过ER机制在富含O的IrO2(110)表面产生CO2的温度低于通过LH机制的CO2。因此,我们预测富含 O 的 IrO2 (110) 表面可以加速 CO 氧化反应速率。















































 京公网安备 11010802027423号
京公网安备 11010802027423号