当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microbial Cleavage of C–F Bonds in Two C6 Per- and Polyfluorinated Compounds via Reductive Defluorination
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-29 , DOI: 10.1021/acs.est.0c04483 Yaochun Yu 1, 2 , Kunyang Zhang 2 , Zhong Li 3 , Changxu Ren 1 , Jin Chen 4 , Ying-Hsuan Lin 4, 5 , Jinyong Liu 1 , Yujie Men 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-29 , DOI: 10.1021/acs.est.0c04483 Yaochun Yu 1, 2 , Kunyang Zhang 2 , Zhong Li 3 , Changxu Ren 1 , Jin Chen 4 , Ying-Hsuan Lin 4, 5 , Jinyong Liu 1 , Yujie Men 1, 2
Affiliation
|
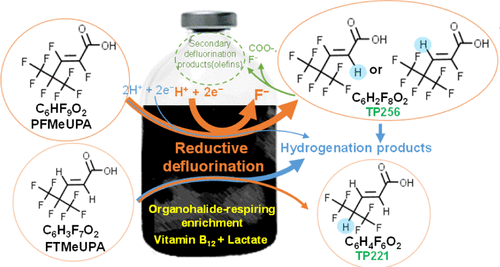
The C–F bond is one of the strongest single bonds in nature. Although microbial reductive dehalogenation is well known for the other organohalides, no microbial reductive defluorination has been documented for perfluorinated compounds except for a single, nonreproducible study on trifluoroacetate. Here, we report on C–F bond cleavage in two C6 per- and polyfluorinated compounds via reductive defluorination by an organohalide-respiring microbial community. The reductive defluorination was demonstrated by the release of F– and the formation of the corresponding product when lactate was the electron donor, and the fluorinated compound was the sole electron acceptor. The major dechlorinating species in the seed culture, Dehalococcoides, were not responsible for the defluorination as no growth of Dehalococcoides or active expression of Dehalococcoides-reductive dehalogenases was observed. It suggests that minor phylogenetic groups in the community might be responsible for the reductive defluorination. These findings expand our fundamental knowledge of microbial reductive dehalogenation and warrant further studies on the enrichment, identification, and isolation of responsible microorganisms and enzymes. Given the wide use and emerging concerns of fluorinated organics (e.g., per- and polyfluoroalkyl substances), particularly the perfluorinated ones, the discovery of microbial defluorination under common anaerobic conditions may provide valuable insights into the environmental fate and potential bioremediation strategies of these notorious contaminants.
中文翻译:

通过还原性脱氟对两种C 6全氟化和多氟化化合物中的CF键进行微生物裂解
C-F键是自然界中最强的单键之一。尽管微生物还原性脱卤对于其他有机卤化物是众所周知的,但除单项不可重复的三氟乙酸盐研究外,全氟化化合物均未见微生物还原性脱氟化作用。在这里,我们报道了通过呼吸有机卤化物的微生物群落的还原性脱氟化作用,在两个C 6全氟化和多氟化化合物中的CF键断裂。还原脱氟是由F的释放证明-和相应的产物的形成时乳酸盐电子给体,和含氟化合物是唯一的电子受体。种子培养中的主要脱氯物种Dehalococcoides,不负责脱氟作为不生长Dehalococcoides或活性表达Dehalococcoides-观察到还原性脱卤素酶。这表明该社区中的少数系统发育群体可能是还原性脱氟的原因。这些发现扩展了我们对微生物还原脱卤的基础知识,并保证了对负责任的微生物和酶的富集,鉴定和分离的进一步研究。鉴于氟化有机物(例如,全氟和多氟烷基物质),尤其是全氟化物的广泛使用和新出现的问题,在常见厌氧条件下发现微生物脱氟可为了解环境命运和这些臭名昭著的污染物的潜在生物修复策略提供有价值的见解。 。
更新日期:2020-11-17
中文翻译:

通过还原性脱氟对两种C 6全氟化和多氟化化合物中的CF键进行微生物裂解
C-F键是自然界中最强的单键之一。尽管微生物还原性脱卤对于其他有机卤化物是众所周知的,但除单项不可重复的三氟乙酸盐研究外,全氟化化合物均未见微生物还原性脱氟化作用。在这里,我们报道了通过呼吸有机卤化物的微生物群落的还原性脱氟化作用,在两个C 6全氟化和多氟化化合物中的CF键断裂。还原脱氟是由F的释放证明-和相应的产物的形成时乳酸盐电子给体,和含氟化合物是唯一的电子受体。种子培养中的主要脱氯物种Dehalococcoides,不负责脱氟作为不生长Dehalococcoides或活性表达Dehalococcoides-观察到还原性脱卤素酶。这表明该社区中的少数系统发育群体可能是还原性脱氟的原因。这些发现扩展了我们对微生物还原脱卤的基础知识,并保证了对负责任的微生物和酶的富集,鉴定和分离的进一步研究。鉴于氟化有机物(例如,全氟和多氟烷基物质),尤其是全氟化物的广泛使用和新出现的问题,在常见厌氧条件下发现微生物脱氟可为了解环境命运和这些臭名昭著的污染物的潜在生物修复策略提供有价值的见解。 。































 京公网安备 11010802027423号
京公网安备 11010802027423号