当前位置:
X-MOL 学术
›
New Biotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Level Production of Amorphadiene using Bacillus subtilis as an Optimized Terpenoid Cell Factory
New Biotechnology ( IF 4.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.nbt.2020.10.007 Hegar Pramastya 1 , Dan Xue 2 , Ingy I Abdallah 3 , Rita Setroikromo 2 , Wim J Quax 2
New Biotechnology ( IF 4.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.nbt.2020.10.007 Hegar Pramastya 1 , Dan Xue 2 , Ingy I Abdallah 3 , Rita Setroikromo 2 , Wim J Quax 2
Affiliation
|
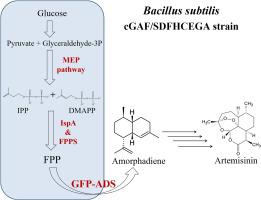
The anti-malarial drug artemisinin, produced naturally in the plant Artemisia annua, experiences unstable and insufficient supply as its production relies heavily on the plant source. To meet the massive demand for this compound, metabolic engineering of microbes has been studied extensively. In this study, we focus on improving the production of amorphadiene, a crucial artemisinin precursor, in Bacillus subtilis. The expression level of the plant-derived amorphadiene synthase (ADS) was upregulated by fusion with green fluorescent protein (GFP). Furthermore, a co-expression system of ADS and a synthetic operon carrying the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway genes was established. Subsequently, farnesyl pyrophosphate synthase (FPPS), a key enzyme in formation of the sesquiterpene precursor farnesyl pyrophosphate (FPP), was expressed to supply sufficient substrate for ADS. The consecutive combination of these features yielded a B. subtilis strain expressing chromosomally integrated GFP-ADS followed by FPPS and a plasmid encoded synthetic operon showing a stepwise increased production of amorphadiene. An experimental design-aided systematic medium optimization was used to maximize the production level for the most promising engineered B. subtilis strain, resulting in an amorphadiene yield of 416 ± 15 mg/L, which is 20-fold higher than that previously reported in B. subtilis and more than double the production in Escherichia coli or Saccharomyces cerevisiae on a shake flask fermentation level.
中文翻译:

使用枯草芽孢杆菌作为优化的萜类细胞工厂高水平生产 Amorphadiene
抗疟药青蒿素是在青蒿中天然产生的,由于其生产严重依赖植物来源,因此供应不稳定且供应不足。为了满足对这种化合物的大量需求,微生物的代谢工程得到了广泛的研究。在这项研究中,我们专注于提高枯草芽孢杆菌中重要的青蒿素前体——紫质二烯的产量。通过与绿色荧光蛋白 (GFP) 融合,植物来源的 amorphadiene 合酶 (ADS) 的表达水平被上调。此外,还建立了 ADS 和携带 2-C-甲基-D-赤藓糖醇-4-磷酸 (MEP) 通路基因的合成操纵子的共表达系统。随后,法呢基焦磷酸合酶 (FPPS),一种形成倍半萜前体法呢基焦磷酸 (FPP) 的关键酶,表达为 ADS 提供足够的底物。这些特征的连续组合产生了表达染色体整合的 GFP-ADS 的枯草芽孢杆菌菌株,随后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。subtilis 菌株表达染色体整合的 GFP-ADS,随后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。subtilis 菌株表达染色体整合的 GFP-ADS,然后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。
更新日期:2021-01-01
中文翻译:

使用枯草芽孢杆菌作为优化的萜类细胞工厂高水平生产 Amorphadiene
抗疟药青蒿素是在青蒿中天然产生的,由于其生产严重依赖植物来源,因此供应不稳定且供应不足。为了满足对这种化合物的大量需求,微生物的代谢工程得到了广泛的研究。在这项研究中,我们专注于提高枯草芽孢杆菌中重要的青蒿素前体——紫质二烯的产量。通过与绿色荧光蛋白 (GFP) 融合,植物来源的 amorphadiene 合酶 (ADS) 的表达水平被上调。此外,还建立了 ADS 和携带 2-C-甲基-D-赤藓糖醇-4-磷酸 (MEP) 通路基因的合成操纵子的共表达系统。随后,法呢基焦磷酸合酶 (FPPS),一种形成倍半萜前体法呢基焦磷酸 (FPP) 的关键酶,表达为 ADS 提供足够的底物。这些特征的连续组合产生了表达染色体整合的 GFP-ADS 的枯草芽孢杆菌菌株,随后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。subtilis 菌株表达染色体整合的 GFP-ADS,随后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。subtilis 菌株表达染色体整合的 GFP-ADS,然后是 FPPS 和质粒编码的合成操纵子,显示出逐步增加的 amorphadiene 产量。使用实验设计辅助的系统培养基优化来最大限度地提高最有前途的工程枯草芽孢杆菌菌株的生产水平,从而获得 416 ± 15 mg/L 的紫质二烯产量,比之前在 B. subtilis 中报道的产量高 20 倍. subtilis 和在摇瓶发酵水平上的大肠杆菌或酿酒酵母产量的两倍以上。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号