当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
InBr3‐Catalyzed Coupling Reaction between Electron‐Deficient Alkenyl Ethers with Silyl Enolates for Stereoselective Synthesis of 1,5‐Dioxo‐alk‐2‐enes
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-10-27 , DOI: 10.1002/ejoc.202001342 Shuichi Nakao 1 , Miki Saikai 1 , Yoshihiro Nishimoto 1 , Makoto Yasuda 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-10-27 , DOI: 10.1002/ejoc.202001342 Shuichi Nakao 1 , Miki Saikai 1 , Yoshihiro Nishimoto 1 , Makoto Yasuda 1
Affiliation
|
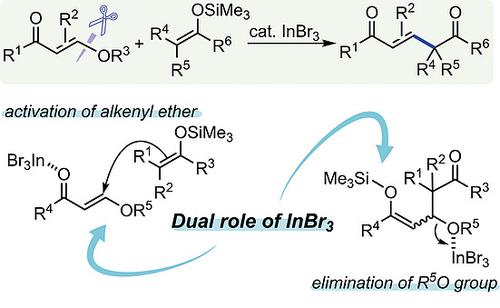
InBr3 catalysis enables the coupling between 2‐carbonylalkenyl ethers and silyl enolates to give 1,5‐dioxo‐alk‐2‐enes featuring alkene moieties with perfect stereoselectivity. Various types of 2‐carbonylalkenyl ethers and silyl enolates are applicable to the present reaction. InBr3 with moderate Lewis acidity plays an important role in both the activation of alkenyl ethers and in the elimination of alkoxy groups regardless of the presence of various coordinative functional groups.

中文翻译:

InBr3催化的电子不足的烯基醚与甲硅烷基烯醇酸酯的立体选择性合成1,5-二氧代烷-2-烯
InBr 3催化使2羰基链烯基醚和甲硅烷基烯醇酸酯之间偶联,可得到具有理想立体选择性的具有烯烃部分的1,5-二氧代烷-2-烯。各种类型的2-羰基烯基醚和甲硅烷基烯醇盐都可用于本反应。路易斯酸度适中的InBr 3在烯基醚的活化和烷氧基的消除中都起着重要的作用,而与各种配位官能团的存在无关。

更新日期:2021-01-05

中文翻译:

InBr3催化的电子不足的烯基醚与甲硅烷基烯醇酸酯的立体选择性合成1,5-二氧代烷-2-烯
InBr 3催化使2羰基链烯基醚和甲硅烷基烯醇酸酯之间偶联,可得到具有理想立体选择性的具有烯烃部分的1,5-二氧代烷-2-烯。各种类型的2-羰基烯基醚和甲硅烷基烯醇盐都可用于本反应。路易斯酸度适中的InBr 3在烯基醚的活化和烷氧基的消除中都起着重要的作用,而与各种配位官能团的存在无关。












































 京公网安备 11010802027423号
京公网安备 11010802027423号