当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ordered Hydrogen-Bonded Alcohol Networks Confined in Lewis Acid Zeolites Accelerate Transfer Hydrogenation Turnover Rates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-27 , DOI: 10.1021/jacs.0c09825 John R. Di Iorio 1 , Blake A. Johnson 1 , Yuriy Román-Leshkov 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-27 , DOI: 10.1021/jacs.0c09825 John R. Di Iorio 1 , Blake A. Johnson 1 , Yuriy Román-Leshkov 1
Affiliation
|
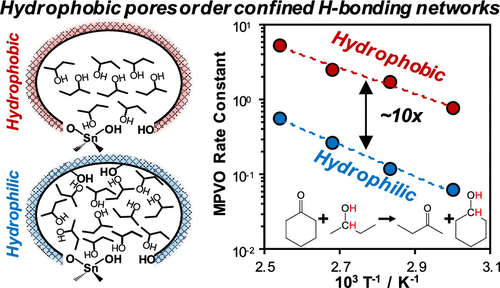
The disruption of ordered water molecules confined within hydrophobic reaction pockets alters the energetics of adsorption and catalysis, but a mechanistic understanding of how nonaqueous solvents influence catalysis in microporous voids remains unclear. Here, we use kinetic analyses coupled with IR spectroscopy to study how alkanol hydrogen-bonding networks confined within hydrophobic and hydrophilic zeolite catalysts modify reaction free energy landscapes. Hydrophobic Beta zeolites containing framework Sn atoms catalyze the transfer hydrogenation reaction of cyclohexanone in a 2-butanol solvent 10× faster than their hydrophilic analogues. This rate enhancement stems from the ability of hydrophobic Sn-Beta to inhibit the formation of extended liquid-like 2-butanol oligomers and promote dimeric H-bonded 2-butanol networks. These different intraporous 2-butanol solvent structures manifest as differences in the activation and adsorption enthalpies and entropies that comprise the free energy landscape of transfer hydrogenation catalysis. The ordered H-bonding solvent network present in hydrophobic Sn-Beta stabilizes the transfer hydrogenation transition state to a greater extent than the liquid-like 2-butanol solvent present in hydrophilic Sn-Beta, giving rise to higher turnover rates on hydrophobic Sn-Beta. Additionally, reactant adsorption within hydrophobic Sn-Beta is driven by the breakup of intraporous solvent-solvent interactions, resulting in positive enthalpies of adsorption that are partially compensated by an increase in the solvent reorganization entropy. Collectively, these results emphasize the ability of the zeolite pore to regulate the structure of confined nonaqueous H-bonding solvent networks, which offers an additional dimension to modulate adsorption and reactivity.
中文翻译:

路易斯酸沸石中的有序氢键醇网络加速转移加氢转化率
限制在疏水反应袋内的有序水分子的破坏改变了吸附和催化的能量学,但对非水溶剂如何影响微孔空隙中的催化作用的机制理解仍不清楚。在这里,我们使用动力学分析结合红外光谱来研究限制在疏水和亲水沸石催化剂内的烷醇氢键网络如何改变反应自由能景观。含有骨架 Sn 原子的疏水性 Beta 沸石在 2-丁醇溶剂中催化环己酮的转移氢化反应比其亲水性类似物快 10 倍。这种速率提高源于疏水性 Sn-Beta 抑制扩展的液体状 2-丁醇低聚物形成并促进二聚 H 键合 2-丁醇网络的能力。这些不同的内孔 2-丁醇溶剂结构表现为活化和吸附焓和熵的差异,这些焓和熵构成了转移氢化催化的自由能景观。疏水性 Sn-Beta 中存在的有序 H 键溶剂网络比亲水性 Sn-Beta 中存在的液体状 2-丁醇溶剂在更大程度上稳定了转移氢化过渡态,从而提高了疏水性 Sn-Beta 的转换率. 此外,疏水性 Sn-Beta 中的反应物吸附是由孔隙内溶剂 - 溶剂相互作用的分解驱动的,导致正吸附焓被溶剂重组熵的增加部分补偿。总的来说,
更新日期:2020-10-27
中文翻译:

路易斯酸沸石中的有序氢键醇网络加速转移加氢转化率
限制在疏水反应袋内的有序水分子的破坏改变了吸附和催化的能量学,但对非水溶剂如何影响微孔空隙中的催化作用的机制理解仍不清楚。在这里,我们使用动力学分析结合红外光谱来研究限制在疏水和亲水沸石催化剂内的烷醇氢键网络如何改变反应自由能景观。含有骨架 Sn 原子的疏水性 Beta 沸石在 2-丁醇溶剂中催化环己酮的转移氢化反应比其亲水性类似物快 10 倍。这种速率提高源于疏水性 Sn-Beta 抑制扩展的液体状 2-丁醇低聚物形成并促进二聚 H 键合 2-丁醇网络的能力。这些不同的内孔 2-丁醇溶剂结构表现为活化和吸附焓和熵的差异,这些焓和熵构成了转移氢化催化的自由能景观。疏水性 Sn-Beta 中存在的有序 H 键溶剂网络比亲水性 Sn-Beta 中存在的液体状 2-丁醇溶剂在更大程度上稳定了转移氢化过渡态,从而提高了疏水性 Sn-Beta 的转换率. 此外,疏水性 Sn-Beta 中的反应物吸附是由孔隙内溶剂 - 溶剂相互作用的分解驱动的,导致正吸附焓被溶剂重组熵的增加部分补偿。总的来说,































 京公网安备 11010802027423号
京公网安备 11010802027423号