当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azido-Cyanide Mixed-Bridged FeIII–NiII Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.inorgchem.0c01917 Jiong Yang 1 , Xin-Hua Zhao 1 , Yi-Fei Deng 1 , Xin-Yu Zhang 1 , Xiao-Yong Chang 1 , Zhiping Zheng 1 , Yuan-Zhu Zhang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-10-26 , DOI: 10.1021/acs.inorgchem.0c01917 Jiong Yang 1 , Xin-Hua Zhao 1 , Yi-Fei Deng 1 , Xin-Yu Zhang 1 , Xiao-Yong Chang 1 , Zhiping Zheng 1 , Yuan-Zhu Zhang 1
Affiliation
|
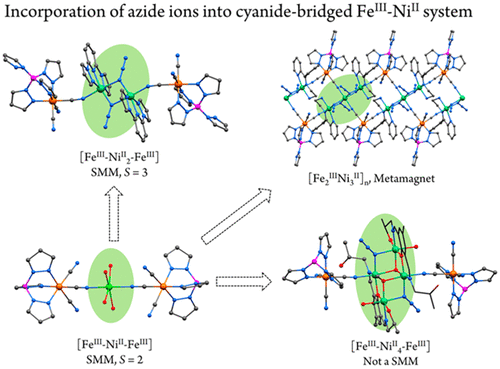
The successful introduction of azide ions as secondary bridges into the FeIII–NiII cyanide system afforded two clusters and one unique 4(3),2-ribbon chain: [(bpzpy)2Ni2(μ2-1,1-N3)2{(pzTp)Fe(CN)3}2]·3H2O [1; bpzpy = 2,6-bis(pyrazol-1-yl)pyridine, and pzTp = tetrakis(pyrazolyl)borate], [(L1)2Ni4(μ3-1,1,1-OCH3)2(μ2-1,1-N3)2(H2O)2{(Tp)Fe(CN)3}2]·2CH3OH·H2O [2; Tp = hydrotris(pyrazolyl)borate, and HL1 = 2,6-bis{(2-hydroxypropylimino)methyl}-4-methylphenol], and [(L2)2Ni3(μ2-1,1-N3)4{(pzTp)Fe(CN)3}2]n (3; L2 = 2-{[phenyl(pyridin-2-yl)methylene]amino}ethan-1-amine). Both 1 and 2 feature the centrosymmetric {FeIII–NiII2–FeIII} and {FeIII–NiII4–FeIII} rodlike structures in which the two peripheral [(TpR)Fe(CN)3]− anions act as monodentate ligands via one cyanide group to link the central azide-bridged [Ni2] and [Ni4] subunit, respectively, while 3 displays an extended structure of the double-zigzag (4,2-ribbon) chain in which the double end-on azide-bridged trinuclear [Ni3] subunits serve as the 4-connected nodes. Magnetic study revealed that intramolecular ferromagnetic coupling is dominated by the azide or cyanide bridges in all of the complexes. Remarkably, complex 1 behaves as a single-molecule magnet with an effective energy barrier of 16.5 cm–1 at zero dc field, while complex 3 exhibits metamagnetism with a hidden spin canting property below 12 K.
中文翻译:

叠氮基氰化物混合式Fe III -Ni II络合物
成功引入叠氮化物离子作为辅助桥进入的Fe III -Ni II得到两个簇和一个独特4(3),氰化物系统2-色带链:[(bpzpy)2的Ni 2(μ 2 -1,1-N 3)2 {(pzTp)Fe(CN)3 } 2 ]·3H 2 O [ 1 ; bpzpy = 2,6-双(吡唑-1-基)吡啶和pzTp =四(吡唑基)硼酸盐],[(L1)2的Ni 4(μ 3 -1,1,1-OCH 3)2(μ 2 -1,1-N 3)2(H 2 O)2 {(Tp)Fe(CN)3 } 2 ]·2CH 3 OH·H 2 O [ 2 ; TP =氢三(吡唑基)硼酸盐,和HL1 = 2,6-双{(2- hydroxypropylimino)甲基} -4-甲基苯酚],和[(L2)2的Ni 3(μ 2 -1,1-N 3)4 {(pzTp)Fe(CN)3 } 2 ] n(3; L 2 = 2-{[苯基(吡啶-2-基亚甲基)亚氨基]氨基}乙-1-胺)。既1和2的特征的中心对称的Fe { III -Ni II 2 -Fe III }和{的Fe III–Ni II 4 –Fe III }棒状结构,其中两个外围的[(Tp R)Fe(CN)3 ] -阴离子通过一个氰化物基团作为单齿配体连接中央叠氮化物桥[Ni 2 ]和[Ni 4分别表示亚基,而3表示双曲折(4,2-碳带)链的扩展结构,其中双末端叠氮桥联的三核[Ni 3 ]亚基用作4个连接的节点。磁性研究表明,在所有配合物中,叠氮化物或氰化物桥决定了分子内铁磁耦合。明显地,复数1表现为在零直流磁场下具有16.5 cm –1的有效能垒的单分子磁体,而复合物3表现出亚磁性,并具有低于12 K的隐藏自旋倾斜特性。
更新日期:2020-11-16
中文翻译:

叠氮基氰化物混合式Fe III -Ni II络合物
成功引入叠氮化物离子作为辅助桥进入的Fe III -Ni II得到两个簇和一个独特4(3),氰化物系统2-色带链:[(bpzpy)2的Ni 2(μ 2 -1,1-N 3)2 {(pzTp)Fe(CN)3 } 2 ]·3H 2 O [ 1 ; bpzpy = 2,6-双(吡唑-1-基)吡啶和pzTp =四(吡唑基)硼酸盐],[(L1)2的Ni 4(μ 3 -1,1,1-OCH 3)2(μ 2 -1,1-N 3)2(H 2 O)2 {(Tp)Fe(CN)3 } 2 ]·2CH 3 OH·H 2 O [ 2 ; TP =氢三(吡唑基)硼酸盐,和HL1 = 2,6-双{(2- hydroxypropylimino)甲基} -4-甲基苯酚],和[(L2)2的Ni 3(μ 2 -1,1-N 3)4 {(pzTp)Fe(CN)3 } 2 ] n(3; L 2 = 2-{[苯基(吡啶-2-基亚甲基)亚氨基]氨基}乙-1-胺)。既1和2的特征的中心对称的Fe { III -Ni II 2 -Fe III }和{的Fe III–Ni II 4 –Fe III }棒状结构,其中两个外围的[(Tp R)Fe(CN)3 ] -阴离子通过一个氰化物基团作为单齿配体连接中央叠氮化物桥[Ni 2 ]和[Ni 4分别表示亚基,而3表示双曲折(4,2-碳带)链的扩展结构,其中双末端叠氮桥联的三核[Ni 3 ]亚基用作4个连接的节点。磁性研究表明,在所有配合物中,叠氮化物或氰化物桥决定了分子内铁磁耦合。明显地,复数1表现为在零直流磁场下具有16.5 cm –1的有效能垒的单分子磁体,而复合物3表现出亚磁性,并具有低于12 K的隐藏自旋倾斜特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号