当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Stability Conditions of the Methane + Tetrabutylphosphonium Bromide + Water Semiclathrate Hydrate System in the Presence and Absence of NaCl and/or MgCl2: Experimental Measurements and Thermodynamic Modeling
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.energyfuels.0c02834 Mohammad Pourranjbar 1 , Hassan Pahlavanzadeh 1 , Soheil Zebardast 1 , Amir H. Mohammadi 2
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-10-27 , DOI: 10.1021/acs.energyfuels.0c02834 Mohammad Pourranjbar 1 , Hassan Pahlavanzadeh 1 , Soheil Zebardast 1 , Amir H. Mohammadi 2
Affiliation
|
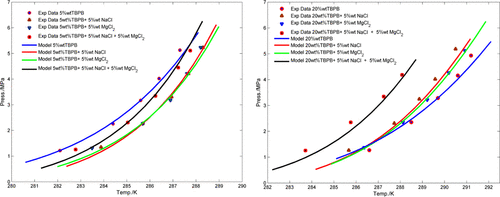
Gas hydrate, or clathrate hydrate, technology is considered to be one of the promising solutions for natural gas storage and transportation. Tetrabutylphosphonium bromide (TBPB) is capable of sorting out stability issues of hydrate formation effectively. On the other hand, using water from natural resources such as sea, river, or well instead of pure water is essential for hydrate formation in the industrial scale. The current study was organized to investigate the hydrate phase behaviors of CH4 + TBPB formed in saline solutions, which are necessary for potential industrial applications. For this purpose, we first generated hydrate dissociation conditions data of the CH4 + TBPB aqueous solution system over TBPB mass fractions of 0.05 and 0.2. It should be mentioned that there is some discrepancy between hydrate dissociation condition data of the CH4 + TBPB + water system reported in literature, which necessitates generation of accurate and reliable data in this work. Afterward, the effects of the presence of NaCl, MgCl2, and NaCl + MgCl2 in aqueous solutions on the hydrate dissociation conditions of the CH4 + TBPB + water system were studied. All equilibrium points were obtained using a reliable constant-volume pressure-search method in the pressure and temperature ranges of 1–5.5 MPa and 280–292 K, respectively. The experimental results show that the aforementioned salts have dual effects on the hydrate phase stability, depending on TBPB concentration in the aqueous solution. In the case of 0.05 mass fraction of TBPB in the aqueous solution, the presence of NaCl (0.05 mass fraction), MgCl2 (0.05 mass fraction), and NaCl + MgCl2 (0.05 + 0.05 mass fractions) can boost the hydrate stability conditions of the CH4 + TBPB + water system and can act as thermodynamic promoters. However, by increasing the TBPB mass fraction in aqueous solution to 0.20 mass fraction, the aforementioned salts can shift the hydrate stability conditions to high pressures/low temperatures and can act as thermodynamic inhibitors. Furthermore, the experimental results show that the thermodynamic inhibition effects of the mineral salts are higher when the pressure of hydrate formation increases. A thermodynamic model based on the van der Waals–Platteeuw (vdW–P) solid solution theory was also developed for the aforementioned systems. The model employs the Soave–Redlich–Kwong equation of state and the Bromley equations for the gas phase and the aqueous phase, respectively. Results show that the thermodynamic model is capable of predicting the hydrate dissociation conditions with acceptable accuracy.
中文翻译:

NaCl和/或MgCl 2存在和不存在下甲烷+溴化四丁基phosph溴化物+水合半水合物水合物系统的相稳定性条件:实验测量和热力学模型
天然气水合物或包合物水合物技术被认为是天然气储存和运输的有前途的解决方案之一。溴化四丁基phosph(TBPB)能够有效解决水合物形成的稳定性问题。另一方面,对于工业规模的水合物形成来说,使用来自海洋,河流或井等自然资源的水代替纯净水是必不可少的。当前的研究是组织来研究盐溶液中形成的CH 4 + TBPB的水合相行为,这对于潜在的工业应用是必需的。为此,我们首先生成了CH 4的水合物解离条件数据+ TBPB水溶液系统的TBPB质量分数为0.05和0.2。应当指出的是,文献报道的CH 4 + TBPB +水系统的水合物解离条件数据之间存在差异,因此有必要在这项工作中生成准确可靠的数据。然后,水溶液中NaCl,MgCl 2和NaCl + MgCl 2的存在对CH 4水合物解离条件的影响+ TBPB +水系统进行了研究。所有平衡点都是使用可靠的恒定体积压力搜索方法分别在1–5.5 MPa和280–292 K的压力和温度范围内获得的。实验结果表明,根据水溶液中TBPB的浓度,上述盐对水合物的相稳定性具有双重影响。在水溶液中TBPB质量分数为0.05的情况下,NaCl(0.05质量分数),MgCl 2(0.05质量分数)和NaCl + MgCl 2(0.05 + 0.05质量分数)的存在可以提高水合物的稳定性条件CH 4的+ TBPB +水系统,可以充当热力学促进剂。然而,通过将水溶液中的TBPB质量分数增加至0.20质量分数,上述盐可将水合物稳定性条件转移至高压/低温并可用作热力学抑制剂。此外,实验结果表明,当水合物形成压力增加时,矿物盐的热力学抑制作用更高。还为上述系统开发了基于范德华兹-普拉特约夫(vdW-P)固溶理论的热力学模型。该模型分别使用Soave–Redlich–Kwong状态方程和Bromley方程分别计算气相和水相。
更新日期:2020-11-19
中文翻译:

NaCl和/或MgCl 2存在和不存在下甲烷+溴化四丁基phosph溴化物+水合半水合物水合物系统的相稳定性条件:实验测量和热力学模型
天然气水合物或包合物水合物技术被认为是天然气储存和运输的有前途的解决方案之一。溴化四丁基phosph(TBPB)能够有效解决水合物形成的稳定性问题。另一方面,对于工业规模的水合物形成来说,使用来自海洋,河流或井等自然资源的水代替纯净水是必不可少的。当前的研究是组织来研究盐溶液中形成的CH 4 + TBPB的水合相行为,这对于潜在的工业应用是必需的。为此,我们首先生成了CH 4的水合物解离条件数据+ TBPB水溶液系统的TBPB质量分数为0.05和0.2。应当指出的是,文献报道的CH 4 + TBPB +水系统的水合物解离条件数据之间存在差异,因此有必要在这项工作中生成准确可靠的数据。然后,水溶液中NaCl,MgCl 2和NaCl + MgCl 2的存在对CH 4水合物解离条件的影响+ TBPB +水系统进行了研究。所有平衡点都是使用可靠的恒定体积压力搜索方法分别在1–5.5 MPa和280–292 K的压力和温度范围内获得的。实验结果表明,根据水溶液中TBPB的浓度,上述盐对水合物的相稳定性具有双重影响。在水溶液中TBPB质量分数为0.05的情况下,NaCl(0.05质量分数),MgCl 2(0.05质量分数)和NaCl + MgCl 2(0.05 + 0.05质量分数)的存在可以提高水合物的稳定性条件CH 4的+ TBPB +水系统,可以充当热力学促进剂。然而,通过将水溶液中的TBPB质量分数增加至0.20质量分数,上述盐可将水合物稳定性条件转移至高压/低温并可用作热力学抑制剂。此外,实验结果表明,当水合物形成压力增加时,矿物盐的热力学抑制作用更高。还为上述系统开发了基于范德华兹-普拉特约夫(vdW-P)固溶理论的热力学模型。该模型分别使用Soave–Redlich–Kwong状态方程和Bromley方程分别计算气相和水相。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号