当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-20 00:00:00 , DOI: 10.1021/acs.jpcc.6b04361 Qing Zhao 1, 2 , Stanley S. H. Ng 1 , Heather J. Kulik 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-20 00:00:00 , DOI: 10.1021/acs.jpcc.6b04361 Qing Zhao 1, 2 , Stanley S. H. Ng 1 , Heather J. Kulik 1
Affiliation
|
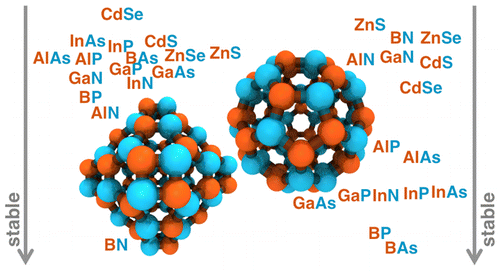
We present a systematic, first-principles study of the role of elemental identity in determining electronic, energetic, and geometric properties of representative A28B28, A30B30, and A36B36 III–V (A = B, Al, Ga, or In and B = N, P, or As) and II–VI (A = Zn or Cd and B = S or Se) fullerene allotropes. A simple descriptor comprising electronegativity differences and covalent radii captures the relative fullerene stability with respect to a nanoparticle reference, and we demonstrate transferability to group IV A72 (A = C, Si, or Ge) fullerenes. We identify the source of relative stability of the four- and six-membered-ring-containing A36B36 and A28B28 fullerene allotropes to the less stable, five-membered-ring-containing A30B30 allotrope. Relative energies of hydrogen-passivated single ring models explain why the even-membered ring structures are typically more stable than the A30B30 fullerene, despite analogies to the well-known C60 allotrope. The ring strain penalty in the four-membered ring is comparable to or smaller than the nonpolar bond penalty in five-membered rings for some materials, and, more importantly, five-membered rings are more numerous in A30B30 than four-membered rings in A36B36 or A28B28 allotropes. Overall, we demonstrate a path forward for predicting the relative stability of fullerene allotropes and isomers of arbitrary shape, size, and elemental composition.
中文翻译:

在整个周期表中预测富勒烯同素异形体的稳定性
我们提出了系统的第一性原理研究,即元素同一性在确定代表性的A 28 B 28,A 30 B 30和A 36 B 36 III–V(A = B,Al ,Ga或In,B = N,P或As)和II–VI(A = Zn或Cd且B = S或Se)富勒烯同素异形体。一个简单的描述符包含电负性差异和共价半径,可以捕获相对于纳米粒子参照物的相对富勒烯稳定性,并且我们证明了向IV A 72组(A = C,Si或Ge)富勒烯的转移性。我们确定了含有四元和六元环的A 36相对稳定性的来源B 36和A 28 B 28富勒烯同素异形体与不太稳定的含五元环的A 30 B 30同素异形体。氢钝化单环模型的相对能量解释了为何偶数环结构通常比A 30 B 30富勒烯更稳定,尽管与众所周知的C 60同素异形体相似。对于某些材料,四元环中的环应变罚分可与五元环中的非极性键罚分相比或小于五元环中的非极性键罚分,更重要的是,A 30 B 30中五元环的数量多于四元环在A 36中响起B 36或A 28 B 28同素异形体。总体而言,我们证明了预测富勒烯同素异形体和任意形状,大小和元素组成的异构体的相对稳定性的途径。
更新日期:2016-07-20
中文翻译:

在整个周期表中预测富勒烯同素异形体的稳定性
我们提出了系统的第一性原理研究,即元素同一性在确定代表性的A 28 B 28,A 30 B 30和A 36 B 36 III–V(A = B,Al ,Ga或In,B = N,P或As)和II–VI(A = Zn或Cd且B = S或Se)富勒烯同素异形体。一个简单的描述符包含电负性差异和共价半径,可以捕获相对于纳米粒子参照物的相对富勒烯稳定性,并且我们证明了向IV A 72组(A = C,Si或Ge)富勒烯的转移性。我们确定了含有四元和六元环的A 36相对稳定性的来源B 36和A 28 B 28富勒烯同素异形体与不太稳定的含五元环的A 30 B 30同素异形体。氢钝化单环模型的相对能量解释了为何偶数环结构通常比A 30 B 30富勒烯更稳定,尽管与众所周知的C 60同素异形体相似。对于某些材料,四元环中的环应变罚分可与五元环中的非极性键罚分相比或小于五元环中的非极性键罚分,更重要的是,A 30 B 30中五元环的数量多于四元环在A 36中响起B 36或A 28 B 28同素异形体。总体而言,我们证明了预测富勒烯同素异形体和任意形状,大小和元素组成的异构体的相对稳定性的途径。















































 京公网安备 11010802027423号
京公网安备 11010802027423号