当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonradical Oxidation of Pollutants with Single-Atom-Fe(III)-Activated Persulfate: Fe(V) Being the Possible Intermediate Oxidant
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-23 , DOI: 10.1021/acs.est.0c04867 Ning Jiang 1, 2 , Haodan Xu 1 , Lihong Wang 1 , Jin Jiang 3 , Tao Zhang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-23 , DOI: 10.1021/acs.est.0c04867 Ning Jiang 1, 2 , Haodan Xu 1 , Lihong Wang 1 , Jin Jiang 3 , Tao Zhang 1
Affiliation
|
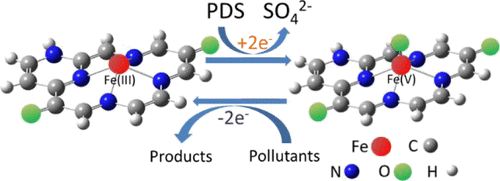
When applied for the remediation of polluted water/soil, peroxydisulfate (PDS) usually needs to be effectively activated to generate sulfate radical as the working oxidant. However, a significant part of the oxidation capacity of PDS is lost in this way because sulfate radical unselectively reacts with most of the substances in water/soil. PDS activation without generating radicals is preferred to maximize its oxidation capacity for targeted pollutants. Here, we report that single-atom Fe(III)- and nitrogen-doped carbon (Fe–N–C) can efficiently activate PDS to selectively remove some organic pollutants following an unreported nonradical pathway. The single-atom Fe(III) coordinated with pyridinic N atoms was confirmed to be the active site for the catalytic decomposition of PDS. However, the PDS decomposition did not produce radicals or reactive oxygen species. It is very likely that the coordinated Fe(III) is readily converted to Fe(V) through two-electron abstraction by PDS, and Fe(V) is responsible for the selective degradation of organic pollutants. The PDS/Fe–N–C-coupled process utilizes more oxidation capacity of PDS than both radical oxidation and other reported nonradical oxidation like PDS/CuO under the same experimental conditions. This process provides a new approach to selectively degrade some organic pollutants through PDS activation.
中文翻译:

单原子铁(III)活化的过硫酸盐对污染物的非自由基氧化:铁(V)是可能的中间氧化剂
当用于修复污水/土壤时,通常需要有效地活化过氧二硫酸盐(PDS)以生成硫酸根自由基作为工作氧化剂。但是,由于硫酸根自由基与水/土壤中的大多数物质发生非选择性反应,因此PDS氧化能力的很大一部分损失了。优选PDS活化而不产生自由基,以最大化其对目标污染物的氧化能力。在这里,我们报道单原子的Fe(III)和氮掺杂的碳(Fe–N–C)可以有效地激活PDS,以遵循未报告的非自由基途径选择性地去除一些有机污染物。与吡啶N原子配位的单原子Fe(III)被证实是PDS催化分解的活性中心。然而,PDS分解不会产生自由基或活性氧。极有可能将配位的Fe(III)通过PDS通过两电子抽象容易地转化为Fe(V),并且Fe(V)负责有机污染物的选择性降解。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。
更新日期:2020-11-03
中文翻译:

单原子铁(III)活化的过硫酸盐对污染物的非自由基氧化:铁(V)是可能的中间氧化剂
当用于修复污水/土壤时,通常需要有效地活化过氧二硫酸盐(PDS)以生成硫酸根自由基作为工作氧化剂。但是,由于硫酸根自由基与水/土壤中的大多数物质发生非选择性反应,因此PDS氧化能力的很大一部分损失了。优选PDS活化而不产生自由基,以最大化其对目标污染物的氧化能力。在这里,我们报道单原子的Fe(III)和氮掺杂的碳(Fe–N–C)可以有效地激活PDS,以遵循未报告的非自由基途径选择性地去除一些有机污染物。与吡啶N原子配位的单原子Fe(III)被证实是PDS催化分解的活性中心。然而,PDS分解不会产生自由基或活性氧。极有可能将配位的Fe(III)通过PDS通过两电子抽象容易地转化为Fe(V),并且Fe(V)负责有机污染物的选择性降解。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。在相同的实验条件下,PDS / Fe–N–C耦合过程利用的PDS氧化能力要比自由基氧化和其他报道的非自由基氧化(如PDS / CuO)高。此过程提供了一种通过PDS活化选择性降解某些有机污染物的新方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号