当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From LiNiO2 to Li2NiO3: Synthesis, Structures and Electrochemical Mechanisms in Li-Rich Nickel Oxides
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-10-22 , DOI: 10.1021/acs.chemmater.0c02880
Matteo Bianchini 1, 2 , Alexander Schiele 1 , Simon Schweidler 1 , Sabrina Sicolo 2 , François Fauth 3 , Emmanuelle Suard 4 , Sylvio Indris 5 , Andrey Mazilkin 1, 6 , Peter Nagel 7 , Stefan Schuppler 7 , Michael Merz 7 , Pascal Hartmann 1, 2 , Torsten Brezesinski 1 , Jürgen Janek 1, 8
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-10-22 , DOI: 10.1021/acs.chemmater.0c02880
Matteo Bianchini 1, 2 , Alexander Schiele 1 , Simon Schweidler 1 , Sabrina Sicolo 2 , François Fauth 3 , Emmanuelle Suard 4 , Sylvio Indris 5 , Andrey Mazilkin 1, 6 , Peter Nagel 7 , Stefan Schuppler 7 , Michael Merz 7 , Pascal Hartmann 1, 2 , Torsten Brezesinski 1 , Jürgen Janek 1, 8
Affiliation
|
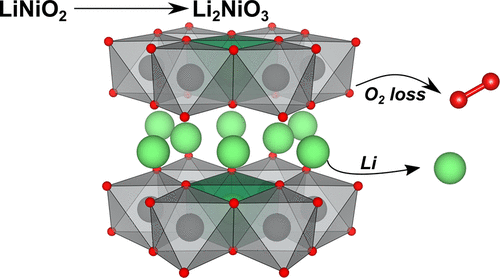
The Li–Ni–O phase diagram contains a variety of compounds, most of which are electrochemically active in Li-ion batteries. Other than the well-known LiNiO2, here we report a facile solid-state method to prepare Li2NiO3 and other Li-rich Ni oxides of composition Li1+xNi1–xO2 (0 ≤ x ≤ 0.33). We characterize their crystal and electronic structure, exhibiting a highly oxidized Ni state and defects of various nature (Li–Ni disorder, stacking faults, oxygen vacancies). We then investigate the use of Li2NiO3 as a cathode active material and show its remarkably high specific capacity, which however fades quickly. While we demonstrate that the initial capacity is due to irreversible O2 release, such process stops quickly in favor of more classical reversible redox mechanisms that allow cycling the material for >100 cycles. After the severe oxygen loss (∼15–20%) and prolonged cycling, the Bragg reflections of Li2NiO3 disappear. Analysis of the diffracted intensities suggests the resulting phase is a disordered rock salt-type material with high Li content, close to Li0.5Ni0.5O, never reported to date and capable of Li diffusion. Our findings demonstrate that the Li–Ni–O phase diagram has not been fully investigated yet, especially concerning the preparation of new promising materials by out-of-equilibrium methods.
中文翻译:

从LiNiO 2到Li 2 NiO 3:富锂氧化镍中的合成,结构和电化学机理
Li-Ni-O相图包含多种化合物,其中大多数在锂离子电池中具有电化学活性。除了公知的LiNiO 2,这里,我们报告一个浅显固态法来制备锂2的NiO 3和组合物中其它栗富Ni氧化物栗1+ X的Ni 1- X Ø 2(0≤ X ≤0.33) 。我们表征了它们的晶体和电子结构,表现出高度氧化的Ni状态和各种性质的缺陷(Li-Ni无序,堆垛层错,氧空位)。然后,我们研究Li 2 NiO 3的使用作为阴极活性材料,它显示出非常高的比容量,但是很快消失。尽管我们证明了初始容量是由于不可逆的O 2释放引起的,但这种过程很快停止了,转而采用了更经典的可逆氧化还原机制,该机制允许材料循环> 100个循环。经过严重的氧气损失(约15–20%)并延长了循环时间,Li 2 NiO 3的布拉格反射消失了。衍射强度分析表明,所得相是一种高锂含量的无序盐岩型材料,接近Li 0.5 Ni 0.5O,迄今从未报道过并且能够扩散Li。我们的发现表明,Li-Ni-O相图尚未得到充分研究,特别是关于通过不平衡方法制备新的有前途的材料。
更新日期:2020-11-12
中文翻译:

从LiNiO 2到Li 2 NiO 3:富锂氧化镍中的合成,结构和电化学机理
Li-Ni-O相图包含多种化合物,其中大多数在锂离子电池中具有电化学活性。除了公知的LiNiO 2,这里,我们报告一个浅显固态法来制备锂2的NiO 3和组合物中其它栗富Ni氧化物栗1+ X的Ni 1- X Ø 2(0≤ X ≤0.33) 。我们表征了它们的晶体和电子结构,表现出高度氧化的Ni状态和各种性质的缺陷(Li-Ni无序,堆垛层错,氧空位)。然后,我们研究Li 2 NiO 3的使用作为阴极活性材料,它显示出非常高的比容量,但是很快消失。尽管我们证明了初始容量是由于不可逆的O 2释放引起的,但这种过程很快停止了,转而采用了更经典的可逆氧化还原机制,该机制允许材料循环> 100个循环。经过严重的氧气损失(约15–20%)并延长了循环时间,Li 2 NiO 3的布拉格反射消失了。衍射强度分析表明,所得相是一种高锂含量的无序盐岩型材料,接近Li 0.5 Ni 0.5O,迄今从未报道过并且能够扩散Li。我们的发现表明,Li-Ni-O相图尚未得到充分研究,特别是关于通过不平衡方法制备新的有前途的材料。

































 京公网安备 11010802027423号
京公网安备 11010802027423号