当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Asymmetric Hydrosulfonylation of 1,3‐Dienes with Sulfonyl Hydrazides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-22 , DOI: 10.1002/anie.202012485 Ming‐Ming Li 1 , Lei Cheng 1 , Li‐Jun Xiao 1 , Jian‐Hua Xie 1 , Qi‐Lin Zhou 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-22 , DOI: 10.1002/anie.202012485 Ming‐Ming Li 1 , Lei Cheng 1 , Li‐Jun Xiao 1 , Jian‐Hua Xie 1 , Qi‐Lin Zhou 1
Affiliation
|
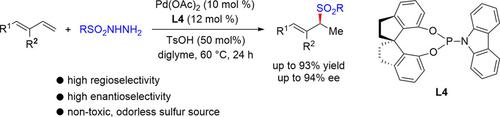
A highly enantio‐ and regioselective hydrosulfonylation of 1,3‐dienes with sulfonyl hydrazides has been realized by using a palladium catalyst containing a monodentate chiral spiro phosphoramidite ligand. The reaction provided an efficient approach to synthetically useful chiral allylic sulfones. Mechanistic studies suggest that the reaction proceeds through the formation of an allyl hydrazine intermediate and subsequent rearrangement to the chiral allylic sulfone product. The transformation of the allyl hydrazine intermediate to the product is the enantioselectivity‐determining step.
中文翻译:

钯催化的磺酰肼与1,3-二烯的不对称加氢磺酰化
通过使用含有单齿手性螺型亚磷酰胺配体的钯催化剂,已实现了1,3二烯与磺酰肼的高度对映和区域选择性的氢磺酰化反应。该反应提供了合成有用的手性烯丙基砜的有效方法。机理研究表明,该反应通过形成烯丙基肼中间体并随后重排为手性烯丙基砜产物而进行。烯丙基肼中间体向产物的转化是确定对映选择性的步骤。
更新日期:2020-10-22
中文翻译:

钯催化的磺酰肼与1,3-二烯的不对称加氢磺酰化
通过使用含有单齿手性螺型亚磷酰胺配体的钯催化剂,已实现了1,3二烯与磺酰肼的高度对映和区域选择性的氢磺酰化反应。该反应提供了合成有用的手性烯丙基砜的有效方法。机理研究表明,该反应通过形成烯丙基肼中间体并随后重排为手性烯丙基砜产物而进行。烯丙基肼中间体向产物的转化是确定对映选择性的步骤。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号