当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acylated 1H-1,2,4-Triazol-5-amines Targeting Human Coagulation Factor XIIa and Thrombin: Conventional and Microscale Synthesis, Anticoagulant Properties, and Mechanism of Action
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-10-22 , DOI: 10.1021/acs.jmedchem.0c01635 Marvin Korff 1 , Lukas Imberg 1 , Jonas M. Will 2 , Nico Bückreiß 3 , Svetlana A. Kalinina 4 , Benjamin M. Wenzel 5 , Gregor A. Kastner 1 , Constantin G. Daniliuc 6 , Maximilian Barth 1 , Ruzanna A. Ovsepyan 7, 8 , Kirill R. Butov 7, 8 , Hans-Ulrich Humpf 4 , Matthias Lehr 1 , Mikhail A. Panteleev 7, 8, 9, 10 , Antti Poso 11, 12 , Uwe Karst 2 , Torsten Steinmetzer 5 , Gerd Bendas 3 , Dmitrii V. Kalinin 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-10-22 , DOI: 10.1021/acs.jmedchem.0c01635 Marvin Korff 1 , Lukas Imberg 1 , Jonas M. Will 2 , Nico Bückreiß 3 , Svetlana A. Kalinina 4 , Benjamin M. Wenzel 5 , Gregor A. Kastner 1 , Constantin G. Daniliuc 6 , Maximilian Barth 1 , Ruzanna A. Ovsepyan 7, 8 , Kirill R. Butov 7, 8 , Hans-Ulrich Humpf 4 , Matthias Lehr 1 , Mikhail A. Panteleev 7, 8, 9, 10 , Antti Poso 11, 12 , Uwe Karst 2 , Torsten Steinmetzer 5 , Gerd Bendas 3 , Dmitrii V. Kalinin 1
Affiliation
|
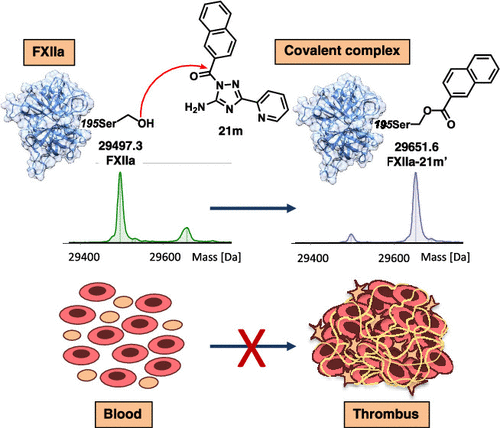
We herein report the conventional and microscale parallel synthesis of selective inhibitors of human blood coagulation factor XIIa and thrombin exhibiting a 1,2,4-triazol-5-amine scaffold. Structural variations of this scaffold allowed identifying derivative 21i, a potent 29 nM inhibitor of FXIIa, with improved selectivity over other tested serine proteases and also finding compound 21m with 27 nM inhibitory activity toward thrombin. For the first time, acylated 1,2,4-triazol-5-amines were proved to have anticoagulant properties and the ability to affect thrombin- and cancer-cell-induced platelet aggregation. Performed mass spectrometric analysis and molecular modeling allowed us to discover previously unknown interactions between the synthesized inhibitors and the active site of FXIIa, which uncovered the mechanistic details of FXIIa inhibition. Synthesized compounds represent a promising starting point for the development of novel antithrombotic drugs or chemical tools for studying the role of FXIIa and thrombin in physiological and pathological processes.
中文翻译:

靶向人类凝血因子XIIa和凝血酶的酰化1 H -1,2,4-三唑-5-胺类化合物:常规和微型合成,抗凝血性质和作用机理
我们在此报告了常规和微观并行合成的人类凝血因子XIIa和凝血酶的选择性抑制剂,这些抑制剂表现出1,2,4-三唑-5-胺骨架。该支架的结构变异允许鉴定衍生物21i(一种有效的FXIIa抑制剂29nM),具有比其他经过测试的丝氨酸蛋白酶更高的选择性,还可以发现化合物21m对凝血酶具有27 nM的抑制活性。首次证明酰化的1,2,4-三唑-5-胺具有抗凝特性,并具有影响凝血酶和癌细胞诱导的血小板凝集的能力。进行的质谱分析和分子建模使我们能够发现合成的抑制剂与FXIIa活性位点之间以前未知的相互作用,从而揭示了FXIIa抑制的机理细节。合成的化合物代表了开发新的抗血栓药物或化学工具以研究FXIIa和凝血酶在生理和病理过程中的作用的有希望的起点。
更新日期:2020-11-12
中文翻译:

靶向人类凝血因子XIIa和凝血酶的酰化1 H -1,2,4-三唑-5-胺类化合物:常规和微型合成,抗凝血性质和作用机理
我们在此报告了常规和微观并行合成的人类凝血因子XIIa和凝血酶的选择性抑制剂,这些抑制剂表现出1,2,4-三唑-5-胺骨架。该支架的结构变异允许鉴定衍生物21i(一种有效的FXIIa抑制剂29nM),具有比其他经过测试的丝氨酸蛋白酶更高的选择性,还可以发现化合物21m对凝血酶具有27 nM的抑制活性。首次证明酰化的1,2,4-三唑-5-胺具有抗凝特性,并具有影响凝血酶和癌细胞诱导的血小板凝集的能力。进行的质谱分析和分子建模使我们能够发现合成的抑制剂与FXIIa活性位点之间以前未知的相互作用,从而揭示了FXIIa抑制的机理细节。合成的化合物代表了开发新的抗血栓药物或化学工具以研究FXIIa和凝血酶在生理和病理过程中的作用的有希望的起点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号