Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Linear Size Contraction of Ligand Protected Ag29 Clusters by Substituting Ag with Cu
ACS Nano ( IF 15.8 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsnano.0c05082 Ananya Baksi 1, 2 , Erik Karsten Schneider 2 , Patrick Weis 2 , Indranath Chakraborty 3 , Olaf Fuhr 1, 4 , Sergei Lebedkin 1, 2 , Wolfgang J. Parak 3 , Manfred M. Kappes 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2020-10-22 , DOI: 10.1021/acsnano.0c05082 Ananya Baksi 1, 2 , Erik Karsten Schneider 2 , Patrick Weis 2 , Indranath Chakraborty 3 , Olaf Fuhr 1, 4 , Sergei Lebedkin 1, 2 , Wolfgang J. Parak 3 , Manfred M. Kappes 1, 2
Affiliation
|
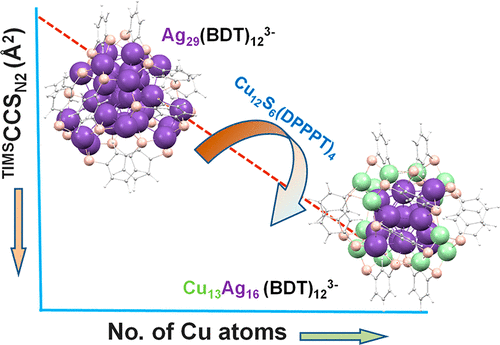
There are only a few examples of atomically precise, ligand protected, bimetallic coinage metal clusters in which molecular structure remains essentially unchanged over a wide composition range starting from the corresponding homometallic species. Such model systems are particularly useful to study the dynamics of alloy formation on the nanoscale. Here we demonstrate the unusual reactivity of solvated metalloid-superatom Ag29(BDT)12(PPh3)4 (BDT = 1,3 benzenedithiol) clusters toward semiconducting Cu12S6(DPPPT)4 (DPPPT = bis(diphenylphosphino)pentane) clusters as an efficient way to exchange multiple copper atoms into the atomically precise silver clusters without changing overall the structure type. Concentration-dependent UV–vis absorption and online mass spectrometry shows that 14 Cu atoms can be exchanged into the silver cluster. Beyond the 14 Cu atom exchange, the cluster degrades to smaller thiolates. Information on cluster structures is obtained from high-resolution ion mobility mass spectrometry, which shows a linear decrease in collision cross section (CCS) with each Ag/Cu exchanged. Several isomeric structures are calculated by density functional theory (DFT), and their calculated collision cross sections are used to identify the most stable isomers for each Ag/Cu exchange product. Ag/Cu exchange is essentially limited to the cluster surface/shell. The core appears not to be involved.
中文翻译:

用铜取代银的配体保护的Ag 29团簇的线性尺寸收缩
仅有几个原子精确的,配体保护的双金属造币金属簇的例子,其中从相应的同金属物种开始,分子结构在很宽的组成范围内基本上保持不变。这样的模型系统对于研究纳米尺度上合金形成的动力学特别有用。在这里,我们证明了溶剂化的准金属-超原子Ag 29(BDT)12(PPh 3)4(BDT = 1,3苯二硫醇)团簇对半导体Cu 12 S 6(DPPPT)4的异常反应性(DPPPT =双(二苯基膦基)戊烷)团簇是将多个铜原子交换为原子精确的银团簇的有效方法,而无需改变整体结构类型。浓度依赖的紫外可见吸收和在线质谱表明,可以将14个铜原子交换成银团簇。除14 Cu原子交换外,团簇降解为较小的硫醇盐。团簇结构的信息是从高分辨率离子迁移质谱法获得的,该质谱图显示了交换的每种Ag / Cu的碰撞截面(CCS)呈线性下降。通过密度泛函理论(DFT)计算了几种同分异构结构,并将其计算出的碰撞截面用于识别每种Ag / Cu交换产物的最稳定的异构体。Ag / Cu交换基本上限于簇表面/壳。核心似乎没有参与。
更新日期:2020-11-25
中文翻译:

用铜取代银的配体保护的Ag 29团簇的线性尺寸收缩
仅有几个原子精确的,配体保护的双金属造币金属簇的例子,其中从相应的同金属物种开始,分子结构在很宽的组成范围内基本上保持不变。这样的模型系统对于研究纳米尺度上合金形成的动力学特别有用。在这里,我们证明了溶剂化的准金属-超原子Ag 29(BDT)12(PPh 3)4(BDT = 1,3苯二硫醇)团簇对半导体Cu 12 S 6(DPPPT)4的异常反应性(DPPPT =双(二苯基膦基)戊烷)团簇是将多个铜原子交换为原子精确的银团簇的有效方法,而无需改变整体结构类型。浓度依赖的紫外可见吸收和在线质谱表明,可以将14个铜原子交换成银团簇。除14 Cu原子交换外,团簇降解为较小的硫醇盐。团簇结构的信息是从高分辨率离子迁移质谱法获得的,该质谱图显示了交换的每种Ag / Cu的碰撞截面(CCS)呈线性下降。通过密度泛函理论(DFT)计算了几种同分异构结构,并将其计算出的碰撞截面用于识别每种Ag / Cu交换产物的最稳定的异构体。Ag / Cu交换基本上限于簇表面/壳。核心似乎没有参与。

































 京公网安备 11010802027423号
京公网安备 11010802027423号