当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into the Radical Chemistry and Product Distribution in the OH-Initiated Oxidation of Benzene
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.est.0c04780 Lu Xu 1 , Kristian H. Møller 2 , John D. Crounse 1 , Henrik G. Kjaergaard 2 , Paul O. Wennberg 1, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.est.0c04780 Lu Xu 1 , Kristian H. Møller 2 , John D. Crounse 1 , Henrik G. Kjaergaard 2 , Paul O. Wennberg 1, 3
Affiliation
|
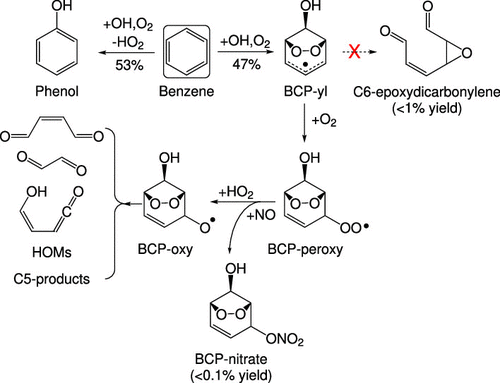
Emissions of aromatic compounds cause air pollution and detrimental health effects. Here, we explore the reaction kinetics and products of key radicals in benzene photo-oxidation. After initial OH addition and reaction with O2, the effective production rates of phenol and bicyclic peroxy radical (BCP-peroxy) are experimentally constrained at 295 K to be 420 ± 80 and 370 ± 70 s–1, respectively. These rates lead to approximately 53% yield for phenol and 47% yield for BCP-peroxy under atmospheric conditions. The reaction of BCP-peroxy with NO produces bicyclic hydroxy nitrate with a branching ratio <0.2%, indicating efficient NOx recycling. Similarly, the reaction of BCP-peroxy with HO2 largely recycles HOx, producing the corresponding bicyclic alkoxy radical (BCP-oxy). Because of the presence of C–C double bonds and multiple functional groups, the chemistry of BCP-oxy and other alkoxy radicals in the system is diverse. Experimental results suggest the aldehydic H-shift and ring-closure to produce an epoxide functionality could be competitive with classic decomposition of alkoxy radicals. These reactions are potential sources of highly oxygenated molecules. Finally, despite the large number of compounds observed in our study, we are unable to account for ∼20% of the carbon flow.
中文翻译:

OH引发的苯氧化中的自由基化学和产物分布的新见解
芳香化合物的排放会导致空气污染和有害的健康影响。在这里,我们探讨了苯光氧化过程中关键自由基的反应动力学和产物。在最初加入OH并与O 2反应之后,苯酚和双环过氧自由基(BCP-peroxy)的有效生产率在295 K上通过实验分别限制为420±80和370±70 s –1。这些速率在大气条件下导致苯酚的产率约为53%,BCP-过氧化合物的产率为47%。BCP-过氧与NO的反应生成支化比<0.2%的双环羟基硝酸盐,表明有效的NO x再循环。同样,BCP-过氧化物与HO 2的反应可大量回收HO x,产生相应的双环烷氧基(BCP-氧基)。由于C–C双键和多个官能团的存在,系统中BCP-氧基和其他烷氧基的化学性质各不相同。实验结果表明,醛基的H移位和闭环反应可产生环氧官能团,与经典的烷氧基分解反应相比具有竞争优势。这些反应是高氧化分子的潜在来源。最后,尽管在我们的研究中观察到了大量的化合物,但我们无法解释约20%的碳流量。
更新日期:2020-11-03
中文翻译:

OH引发的苯氧化中的自由基化学和产物分布的新见解
芳香化合物的排放会导致空气污染和有害的健康影响。在这里,我们探讨了苯光氧化过程中关键自由基的反应动力学和产物。在最初加入OH并与O 2反应之后,苯酚和双环过氧自由基(BCP-peroxy)的有效生产率在295 K上通过实验分别限制为420±80和370±70 s –1。这些速率在大气条件下导致苯酚的产率约为53%,BCP-过氧化合物的产率为47%。BCP-过氧与NO的反应生成支化比<0.2%的双环羟基硝酸盐,表明有效的NO x再循环。同样,BCP-过氧化物与HO 2的反应可大量回收HO x,产生相应的双环烷氧基(BCP-氧基)。由于C–C双键和多个官能团的存在,系统中BCP-氧基和其他烷氧基的化学性质各不相同。实验结果表明,醛基的H移位和闭环反应可产生环氧官能团,与经典的烷氧基分解反应相比具有竞争优势。这些反应是高氧化分子的潜在来源。最后,尽管在我们的研究中观察到了大量的化合物,但我们无法解释约20%的碳流量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号