当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capture of Electrochemically Generated Fleeting Carbazole Radical Cations and Elucidation of Carbazole Dimerization Mechanism by Mass Spectrometry
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.analchem.0c01223 Chengyuan Liu 1, 2 , Qi Wang 1 , Brian E. Hivick 3 , Yongling Ai 1 , Pier Alexandre Champagne 1 , Yang Pan 2 , Hao Chen 1, 3
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.analchem.0c01223 Chengyuan Liu 1, 2 , Qi Wang 1 , Brian E. Hivick 3 , Yongling Ai 1 , Pier Alexandre Champagne 1 , Yang Pan 2 , Hao Chen 1, 3
Affiliation
|
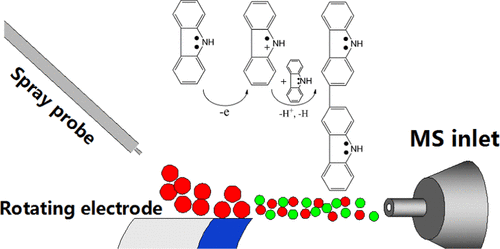
The capture of reactive intermediates is important for the elucidation of reaction mechanisms. We report the first observation of electrochemically generated, short-lived radical cations of carbazole (t1/2 ≈ 97 μs) and two N-substituted carbazole derivatives by mass spectrometry. In addition, online investigation of the reactivity of electrochemically generated carbazole radical cations supports that the carbazole dimerization mechanism involves the reaction of one radical cation with one neutral molecule rather than the previously proposed coupling of two radical cations.
中文翻译:

捕获电化学生成的快速漂移的咔唑自由基阳离子并通过质谱阐明咔唑二聚作用机理
捕获反应性中间体对于阐明反应机理很重要。我们报告了通过质谱法对咔唑(t 1/ 2≈97μs)和两个N-取代的咔唑衍生物进行电化学生成,寿命短的自由基阳离子的首次观察。此外,在线研究电化学产生的咔唑自由基阳离子的反应性表明,咔唑二聚机理涉及一个自由基阳离子与一个中性分子的反应,而不是先前提出的两个自由基阳离子的偶联。
更新日期:2020-12-01
中文翻译:

捕获电化学生成的快速漂移的咔唑自由基阳离子并通过质谱阐明咔唑二聚作用机理
捕获反应性中间体对于阐明反应机理很重要。我们报告了通过质谱法对咔唑(t 1/ 2≈97μs)和两个N-取代的咔唑衍生物进行电化学生成,寿命短的自由基阳离子的首次观察。此外,在线研究电化学产生的咔唑自由基阳离子的反应性表明,咔唑二聚机理涉及一个自由基阳离子与一个中性分子的反应,而不是先前提出的两个自由基阳离子的偶联。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号