当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereocontrolled α-Galactosylation under Cooperative Catalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-10-16 , DOI: 10.1021/acs.joc.0c01279 Melanie Shadrick 1 , Yashapal Singh 1 , Alexei V Demchenko 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-10-16 , DOI: 10.1021/acs.joc.0c01279 Melanie Shadrick 1 , Yashapal Singh 1 , Alexei V Demchenko 1
Affiliation
|
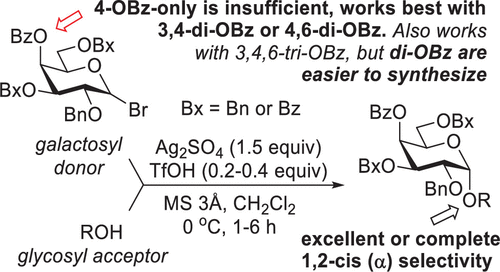
A recent discovery of a cooperative catalysis comprising a silver salt and an acid led to a dramatic improvement in the way glycosyl halides are glycosidated. Excellent yields have been achieved, but the stereoselectivity achieved with 2-O-benzylated donors was poor. Reported herein is our first attempt to refine the stereoselectivity of the cooperatively catalyzed galactosylation reaction. Careful optimization of the reaction conditions along with studying effects of the remote protecting groups led to excellent stereocontrol of α-galactosylation of a variety of glycosyl acceptors with differentially protected galactosyl donors.
中文翻译:

协同催化下的立体控制α-半乳糖基化
最近发现的包含银盐和酸的协同催化使糖基卤化物的糖苷化方式得到了显着改进。已经实现了优异的产率,但使用 2- O-苄基化供体实现的立体选择性较差。本文报道的是我们首次尝试改进协同催化的半乳糖基化反应的立体选择性。仔细优化反应条件并研究远程保护基团的影响,可以对具有差异保护的半乳糖基供体的各种糖基受体的 α-半乳糖基化进行出色的立体控制。
更新日期:2020-12-18
中文翻译:

协同催化下的立体控制α-半乳糖基化
最近发现的包含银盐和酸的协同催化使糖基卤化物的糖苷化方式得到了显着改进。已经实现了优异的产率,但使用 2- O-苄基化供体实现的立体选择性较差。本文报道的是我们首次尝试改进协同催化的半乳糖基化反应的立体选择性。仔细优化反应条件并研究远程保护基团的影响,可以对具有差异保护的半乳糖基供体的各种糖基受体的 α-半乳糖基化进行出色的立体控制。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号