当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radiosynthesis and quality control testing of the tau imaging positron emission tomography tracer [18F]PM‐PBB3 for clinical applications
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-10-17 , DOI: 10.1002/jlcr.3890 Kazunori Kawamura 1 , Hiroki Hashimoto 1 , Kenji Furutsuka 1, 2 , Takayuki Ohkubo 1, 2 , Tomoya Fujishiro 1, 3 , Takahiro Togashi 1, 3 , Daisuke Arashi 1, 3 , Toshiyuki Sakai 1, 3 , Masatoshi Muto 1, 3 , Masanao Ogawa 1, 2 , Yusuke Kurihara 1, 2 , Nobuki Nengaki 1, 2 , Makoto Takei 1 , Kazuyoshi Nemoto 1 , Makoto Higuchi 4 , Ming-Rong Zhang 1
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-10-17 , DOI: 10.1002/jlcr.3890 Kazunori Kawamura 1 , Hiroki Hashimoto 1 , Kenji Furutsuka 1, 2 , Takayuki Ohkubo 1, 2 , Tomoya Fujishiro 1, 3 , Takahiro Togashi 1, 3 , Daisuke Arashi 1, 3 , Toshiyuki Sakai 1, 3 , Masatoshi Muto 1, 3 , Masanao Ogawa 1, 2 , Yusuke Kurihara 1, 2 , Nobuki Nengaki 1, 2 , Makoto Takei 1 , Kazuyoshi Nemoto 1 , Makoto Higuchi 4 , Ming-Rong Zhang 1
Affiliation
|
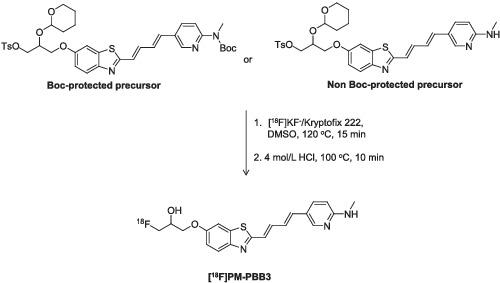
Recently, we produced 11C‐labeled 2‐((1E,3E)‐4‐(6‐(methylamino)pyridin‐3‐yl)buta‐1,3‐dienyl)benzo[d]thiazol‐6‐ol ([11C]PBB3) as a clinically useful positron emission tomography (PET) tracer for in vivo imaging of tau pathologies in the human brain. To overcome the limitations (i.e., rapid in vivo metabolism and short half‐life) of [11C]PBB3, we further synthesized 18F‐labeled 1‐fluoro‐3‐((2‐((1E,3E)‐4‐(6‐(methylamino)pyridine‐3‐yl)buta‐1,3‐dien‐1‐yl)benzo[d]thiazol‐6‐yl)oxy)propan‐2‐ol ([18F]PM‐PBB3). [18F]PM‐PBB3 is also a useful tau PET tracer for imaging tau pathologies. In this study, we developed a routine radiosynthesis and quality control testing of [18F]PM‐PBB3 for clinical applications. [18F]PM‐PBB3 was synthesized by direct 18F‐fluorination of the tosylated derivative, followed by removal of the protecting group. [18F]PM‐PBB3 was obtained with sufficient radioactivity (25 ± 6.0% of the nondecay‐corrected radiochemical yield at the end of synthesis, EOS), radiochemical purity (98 ± 0.6%), and molar activity (350 ± 94 GBq/μmol at EOS; n = 53). Moreover, [18F]PM‐PBB3 consistently retained >95% of radiochemical purity for 60 min without undergoing photoisomerization using a new UV‐cutoff light (yellow light) fixed in the hot cell to monitor the synthesis. All the results of the quality control testing for the [18F]PM‐PBB3 injection complied with our in‐house quality control and quality assurance specifications. We have accomplished >200 production runs of [18F]PM‐PBB3 in our facility for various research purposes.
中文翻译:

tau 成像正电子发射断层扫描示踪剂 [18F]PM-PBB3 临床应用的放射合成和质量控制测试
最近,我们生产了11 C标记的2-((1 E ,3 E )-4-(6-(甲氨基)吡啶-3-基)丁-1,3-二烯基)苯并[ d ]噻唑-6-醇([ 11 C]PBB3) 作为临床上有用的正电子发射断层扫描 (PET) 示踪剂,用于人脑 tau 病理的体内成像。为了克服[ 11 C]PBB3的局限性(即体内代谢快和半衰期短),我们进一步合成了18 F标记的1-氟-3-((2-((1 E ,3 E )- 4-(6-(甲氨基)吡啶-3-基)丁-1,3-二烯-1-基)苯并[ d ]噻唑-6-基)氧基)丙-2-醇([ 18 F]PM-多溴联苯3)。 [ 18 F]PM-PBB3 也是一种有用的 tau PET 示踪剂,用于 tau 病理学成像。在本研究中,我们开发了用于临床应用的 [ 18 F]PM-PBB3 的常规放射合成和质量控制测试。 [ 18 F]PM-PBB3 是通过对甲苯磺酰化衍生物进行直接18 F-氟化,然后去除保护基来合成的。 [ 18 F]PM-PBB3 具有足够的放射性(合成结束时非衰变校正放射化学产率的 25 ± 6.0%,EOS)、放射化学纯度(98 ± 0.6%)和摩尔活度(350 ± 94 GBq /μmol EOS; n = 53)。此外,[ 18 F]PM-PBB3 在 60 分钟内始终保持 >95% 的放射化学纯度,无需使用固定在热室中的新紫外线截止光(黄光)来监测合成,而无需进行光异构化。 [ 18 F]PM-PBB3 注射液的所有质量控制测试结果均符合我们的内部质量控制和质量保证规范。 我们已在我们的设施中完成了 >200 次 [ 18 F]PM-PBB3 生产运行,用于各种研究目的。
更新日期:2020-10-17
中文翻译:

tau 成像正电子发射断层扫描示踪剂 [18F]PM-PBB3 临床应用的放射合成和质量控制测试
最近,我们生产了11 C标记的2-((1 E ,3 E )-4-(6-(甲氨基)吡啶-3-基)丁-1,3-二烯基)苯并[ d ]噻唑-6-醇([ 11 C]PBB3) 作为临床上有用的正电子发射断层扫描 (PET) 示踪剂,用于人脑 tau 病理的体内成像。为了克服[ 11 C]PBB3的局限性(即体内代谢快和半衰期短),我们进一步合成了18 F标记的1-氟-3-((2-((1 E ,3 E )- 4-(6-(甲氨基)吡啶-3-基)丁-1,3-二烯-1-基)苯并[ d ]噻唑-6-基)氧基)丙-2-醇([ 18 F]PM-多溴联苯3)。 [ 18 F]PM-PBB3 也是一种有用的 tau PET 示踪剂,用于 tau 病理学成像。在本研究中,我们开发了用于临床应用的 [ 18 F]PM-PBB3 的常规放射合成和质量控制测试。 [ 18 F]PM-PBB3 是通过对甲苯磺酰化衍生物进行直接18 F-氟化,然后去除保护基来合成的。 [ 18 F]PM-PBB3 具有足够的放射性(合成结束时非衰变校正放射化学产率的 25 ± 6.0%,EOS)、放射化学纯度(98 ± 0.6%)和摩尔活度(350 ± 94 GBq /μmol EOS; n = 53)。此外,[ 18 F]PM-PBB3 在 60 分钟内始终保持 >95% 的放射化学纯度,无需使用固定在热室中的新紫外线截止光(黄光)来监测合成,而无需进行光异构化。 [ 18 F]PM-PBB3 注射液的所有质量控制测试结果均符合我们的内部质量控制和质量保证规范。 我们已在我们的设施中完成了 >200 次 [ 18 F]PM-PBB3 生产运行,用于各种研究目的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号