European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-10-16 , DOI: 10.1016/j.ejmech.2020.112944 Serena Massari , Chiara Bertagnin , Maria Chiara Pismataro , Anna Donnadio , Giulio Nannetti , Tommaso Felicetti , Stefano Di Bona , Maria Giulia Nizi , Leonardo Tensi , Giuseppe Manfroni , Maria Isabel Loza , Stefano Sabatini , Violetta Cecchetti , Jose Brea , Laura Goracci , Arianna Loregian , Oriana Tabarrini
|
Influenza viruses (Flu) are responsible for seasonal epidemics causing high rates of morbidity, which can dramatically increase during severe pandemic outbreaks. Antiviral drugs are an indispensable weapon to treat infected people and reduce the impact on human health, nevertheless anti-Flu armamentarium still remains inadequate.
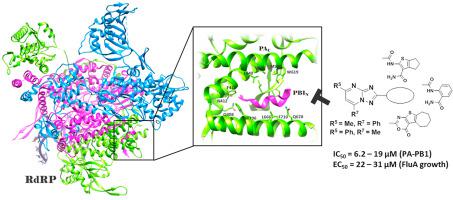
In search for new anti-Flu drugs, our group has focused on viral RNA-dependent RNA polymerase (RdRP) developing disruptors of PA-PB1 subunits interface with the best compounds characterized by cycloheptathiophene-3-carboxamide and 1,2,4-triazolo[1,5-a]pyrimidine-2-carboxamide scaffolds. By merging these moieties, two very interesting hybrid compounds were recently identified, starting from which, in this paper, a series of analogues were designed and synthesized. In particular, a thorough exploration of the cycloheptathiophene-3-carboxamide moiety led to acquire important SAR insight and identify new active compounds showing both the ability to inhibit PA-PB1 interaction and viral replication in the micromolar range and at non-toxic concentrations. For few compounds, the ability to efficiently inhibit PA-PB1 subunits interaction did not translate into anti-Flu activity. Chemical/physical properties were investigated for a couple of compounds suggesting that the low solubility of compound 14, due to a strong crystal lattice, may have impaired its antiviral activity. Finally, computational studies performed on compound 23, in which the phenyl ring suitably replaced the cycloheptathiophene, suggested that, in addition to hydrophobic interactions, H-bonds enhanced its binding within the PAC cavity.
中文翻译:

靶向甲型流感病毒聚合酶PA-PB1界面的1,2,4-三唑并[1,5- a ]嘧啶-2-羧酰胺基化合物的合成与表征
流感病毒(Flu)是造成高发病率的季节性流行病的原因,在严重的大流行病爆发期间,发病率可能急剧上升。抗病毒药物是治疗感染者并减少对人类健康影响的必不可少的武器,尽管如此,抗流感武器库仍然不足。
在寻找新的抗流感药物时,我们小组专注于病毒RNA依赖的RNA聚合酶(RdRP),开发PA-PB1亚基的干扰物,其具有以环庚噻吩-3-羧酰胺和1,2,4-三唑为特征的最佳化合物[1,5- a] pyrimidine-2-carboxamide支架。通过合并这些部分,最近发现了两个非常有趣的杂化化合物,从本文开始,设计并合成了一系列类似物。特别是,对环庚噻吩-3-甲酰胺部分的彻底研究导致获得了重要的SAR信息,并确定了新的活性化合物,这些化合物在微摩尔范围内和无毒浓度下均具有抑制PA-PB1相互作用和病毒复制的能力。对于少数化合物,有效抑制PA-PB1亚基相互作用的能力并未转化为抗Flu活性。研究了两种化合物的化学/物理性质,表明化合物14的溶解度低,由于强大的晶格,可能已削弱其抗病毒活性。最后,对其中苯环适当取代了环庚噻吩的化合物23进行的计算研究表明,除疏水相互作用外,H键还增强了其在PA C腔内的结合。































 京公网安备 11010802027423号
京公网安备 11010802027423号