当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Synthesized SO42–/ZrO2-HZSM-5 Solid Acid Catalyst for Carbamate Decomposition in CO2 Capture
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-15 , DOI: 10.1021/acs.est.0c04946 Lei Xing 1, 2 , Kexin Wei 1, 2 , Qiangwei Li 1, 2 , Rujie Wang 1, 2 , Shihan Zhang 3 , Lidong Wang 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-10-15 , DOI: 10.1021/acs.est.0c04946 Lei Xing 1, 2 , Kexin Wei 1, 2 , Qiangwei Li 1, 2 , Rujie Wang 1, 2 , Shihan Zhang 3 , Lidong Wang 1, 2
Affiliation
|
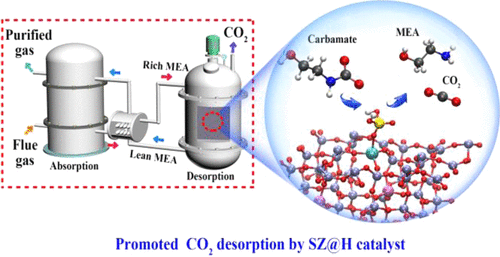
Amine-based CO2 capture technology requires high-energy consumption because the desorption temperature required for carbamate breakdown during absorbent regeneration is higher than 110 °C. In this study, we report a stable solid acid catalyst, namely, SO42–/ZrO2-HZSM-5 (SZ@H), which has improved Lewis acid sites (LASs) and Bronsted acid sites (BASs). The improved LASs and BASs enabled the CO2 desorption temperature to be decreased to less than 98 °C. The BASs and LASs of SZ@H preferred to donate or accept protons; thus, the amount and rate of CO2 desorption from spent monoethanolamine were more than 40 and 37% higher, respectively, when using SZ@H than when not using any catalyst. Consequently, the energy consumption was reduced by approximately 31%. A catalyzed proton-transfer mechanism is proposed for SZ@H-catalyzed CO2 regeneration through experimental characterization and theoretical calculations. The results reveal the role of proton transfer during CO2 desorption, which enables the feasibility of catalysts for CO2 capture in industrial applications.
中文翻译:

一步法合成SO 4 2- /的ZrO 2在CO -HZSM -5-固体酸催化剂用于氨基甲酸盐分解2捕获
基于胺的CO 2捕集技术需要消耗大量的能量,因为吸收剂再生期间氨基甲酸酯分解所需的解吸温度高于110°C。在这项研究中,我们报告了一种稳定的固体酸催化剂,即SO 4 2- / ZrO 2 -HZSM-5(SZ @ H),该催化剂具有改善的路易斯酸位点(LASs)和布朗斯台德酸位点(BASs)。改进的LAS和BAS使CO 2的解吸温度降低到低于98°C。SZ @ H的BAS和LAS倾向于捐赠或接受质子;因此,CO 2的量和速率使用SZ @ H时,用过的单乙醇胺的脱附率分别比不使用任何催化剂时高40%和37%。因此,能耗降低了约31%。通过实验表征和理论计算,提出了SZ @ H催化CO 2再生的催化质子转移机理。结果揭示了质子转移在CO 2解吸过程中的作用,这使催化剂在工业应用中捕获CO 2的可行性成为可能。
更新日期:2020-10-15
中文翻译:

一步法合成SO 4 2- /的ZrO 2在CO -HZSM -5-固体酸催化剂用于氨基甲酸盐分解2捕获
基于胺的CO 2捕集技术需要消耗大量的能量,因为吸收剂再生期间氨基甲酸酯分解所需的解吸温度高于110°C。在这项研究中,我们报告了一种稳定的固体酸催化剂,即SO 4 2- / ZrO 2 -HZSM-5(SZ @ H),该催化剂具有改善的路易斯酸位点(LASs)和布朗斯台德酸位点(BASs)。改进的LAS和BAS使CO 2的解吸温度降低到低于98°C。SZ @ H的BAS和LAS倾向于捐赠或接受质子;因此,CO 2的量和速率使用SZ @ H时,用过的单乙醇胺的脱附率分别比不使用任何催化剂时高40%和37%。因此,能耗降低了约31%。通过实验表征和理论计算,提出了SZ @ H催化CO 2再生的催化质子转移机理。结果揭示了质子转移在CO 2解吸过程中的作用,这使催化剂在工业应用中捕获CO 2的可行性成为可能。































 京公网安备 11010802027423号
京公网安备 11010802027423号