Toxicology and Applied Pharmacology ( IF 3.3 ) Pub Date : 2020-10-15 , DOI: 10.1016/j.taap.2020.115285 Joshua T. Gamse , Wendy Freebern , Rashade Haynes II , Frank Simutis , Mary Pazian , James Crona , Helen G. Haggerty , Michael Graziano , Roderick Todd Bunch
|
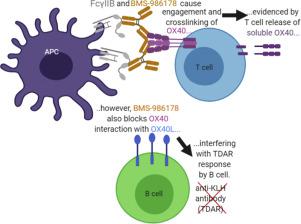
The OX40 receptor plays a crucial co-stimulatory role in T effector cell survival, expansion, cytokine production, and cytotoxicity to tumor cells; therefore, OX40 agonists are being evaluated as anti-cancer immunotherapies, especially in combination with checkpoint inhibitors. To support clinical development of BMS-986178 (an OX40 agonist antibody), two repeat-dose toxicity studies were conducted in cynomolgus monkeys. In the first study, BMS-986178 was administered intravenously (IV) once weekly for one month at doses from 30 to 120 mg/kg. BMS-986178 was well tolerated; surprisingly, immune function was suppressed rather than increased based on pharmacodynamic (PD) and flow cytometry readouts (e.g. T-cell dependent antibody response [TDAR]). To determine whether immune suppression was due to a bi-phasic response, a follow-up study was conducted at lower doses (1 and 10 mg/kg). Although receptor engagement was confirmed, immune function was still suppressed at both doses. In addition, treatment-emergent anti-drug antibodies (ADAs) at 1 mg/kg resulted in hypersensitivity reactions and reduced BMS-986178 exposure after repeated dosing, which precluded a full PD assessment at this dose. In conclusion, BMS-986178 was clinically well-tolerated by monkeys at weekly IV doses from 10 to 120 mg/kg (AUC[0–168] ≤ 712,000 μg●h/mL). However, despite target engagement, PD assays and other immune endpoints demonstrated immune suppression, not stimulation. Due to the inverted immune response at higher doses and the onset of ADAs, additional repeat-dose toxicity studies of BMS-986178 in monkeys (that would typically be required to support Phase 3 clinical trials and registration) would not add value for human safety assessment.
中文翻译:

施用人类T效应细胞激动剂(OX40)抗体的猴子的免疫应答降低
OX40受体在T效应细胞的存活,扩增,细胞因子产生和对肿瘤细胞的细胞毒性中起着至关重要的共刺激作用。因此,OX40激动剂正在被评估为抗癌免疫疗法,尤其是与检查点抑制剂联合使用时。为支持BMS-986178(OX40激动剂抗体)的临床开发,在食蟹猴中进行了两次重复剂量毒性研究。在第一个研究中,BMS-986178每周一次静脉内(IV)施用,剂量为30至120 mg / kg,一个月。BMS-986178的耐受性良好;令人惊讶的是,基于药效学(PD)和流式细胞仪读数(例如,T细胞依赖性抗体应答[TDAR]。为了确定免疫抑制是否是由于双相反应引起的,以较低剂量(1和10 mg / kg)进行了随访研究。尽管已确认受体参与,但两种剂量的免疫功能仍被抑制。此外,在重复给药后,治疗中出现的1 mg / kg的抗药性抗体(ADAs)导致超敏反应并减少BMS-986178的暴露,因此无法对此剂量进行完整的PD评估。总之,猴的BMS-986178在临床上具有很好的耐受性,每周IV剂量为10至120 mg / kg(AUC [0–168]≤712,000μg·h / mL)。然而,尽管有靶点参与,PD分析和其他免疫终点仍显示出免疫抑制作用,而非刺激作用。由于高剂量的反向免疫反应和ADA的发作,

































 京公网安备 11010802027423号
京公网安备 11010802027423号