当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Amide N–C Hiyama Cross-Coupling: Synthesis of Ketones
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-14 , DOI: 10.1021/acs.orglett.0c03260 Muhammad Aliyu Idris 1 , Sunwoo Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-14 , DOI: 10.1021/acs.orglett.0c03260 Muhammad Aliyu Idris 1 , Sunwoo Lee 1
Affiliation
|
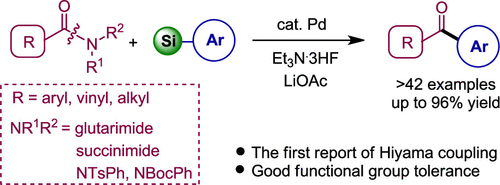
N-Acylglutarimides and arylsiloxanes reacted in the presence of Pd(OAc)2/PCy3, Et3N·3HF, and LiOAc to provide the corresponding arylketones in good yields. Aryl-, vinyl-, and alkyl-substituted N-acylglutarimides showed good activity in the coupling reactions of arylsiloxanes. The reaction had a broad substrate scope and showed good functional group tolerance. N-Benzoylsuccinimide and N-protected N-phenylbenzamides showed good activities in coupling reactions with phenylsiloxane. The employment of CuF2 as an activor afforded the decarbonylative products at 160 °C.
中文翻译:

钯催化的酰胺NC Hiyama交叉偶联:酮的合成
N-酰基戊二酰亚胺和芳基硅氧烷在Pd(OAc)2 / PCy 3,Et 3 N·3HF和LiOAc的存在下反应,以高收率提供相应的芳基酮。芳基,乙烯基和烷基取代的N-酰基戊二酰亚胺在芳基硅氧烷的偶联反应中显示出良好的活性。该反应具有广泛的底物范围并且显示出良好的官能团耐受性。N-苯甲酰基琥珀酰亚胺和N-保护的N-苯基苯甲酰胺在与苯基硅氧烷的偶联反应中显示出良好的活性。使用CuF 2作为活化剂在160℃提供了脱羰基产物。
更新日期:2020-12-04
中文翻译:

钯催化的酰胺NC Hiyama交叉偶联:酮的合成
N-酰基戊二酰亚胺和芳基硅氧烷在Pd(OAc)2 / PCy 3,Et 3 N·3HF和LiOAc的存在下反应,以高收率提供相应的芳基酮。芳基,乙烯基和烷基取代的N-酰基戊二酰亚胺在芳基硅氧烷的偶联反应中显示出良好的活性。该反应具有广泛的底物范围并且显示出良好的官能团耐受性。N-苯甲酰基琥珀酰亚胺和N-保护的N-苯基苯甲酰胺在与苯基硅氧烷的偶联反应中显示出良好的活性。使用CuF 2作为活化剂在160℃提供了脱羰基产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号