当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MOF-74-M (M = Mn, Co, Ni, Zn, MnCo, MnNi, and MnZn) for Low-Temperature NH3-SCR and In Situ DRIFTS Study Reaction Mechanism
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-10-13 , DOI: 10.1021/acsami.0c11035 Shangzhi Xie 1 , Qiuju Qin 1 , Hao Liu 1 , Lijian Jin 1 , Xiaoling Wei 1 , Jiaxing Liu 1 , Xia Liu 1 , Yinchao Yao 1 , Lihui Dong 1 , Bin Li 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-10-13 , DOI: 10.1021/acsami.0c11035 Shangzhi Xie 1 , Qiuju Qin 1 , Hao Liu 1 , Lijian Jin 1 , Xiaoling Wei 1 , Jiaxing Liu 1 , Xia Liu 1 , Yinchao Yao 1 , Lihui Dong 1 , Bin Li 1
Affiliation
|
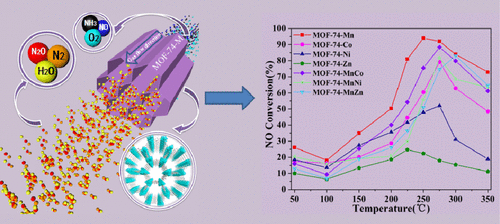
Monometallic and bimetallic MOF-74-M (M = Mn, Co, Ni, Zn, MnCo, MnNi, and MnZn) catalysts were prepared by the solvothermal method for NH3-SCR. XRD, BET, SEM, and EDS-mapping tests indicate the successful synthesis of the MOF-74-M catalyst with uniform distribution of metal elements and large specific surface area, and the morphology is almost hexagonal. Adding Mn element to a single-metal catalyst can enhance activity, which is mainly because of the existence of various valence states of Mn so that it has excellent redox properties; the catalytic activity of water and sulfur resistance tests showed that the catalytic activity of MOF-74-M increases after adding a proper amount of SO2, mainly because of the increase in acidic sites. In situ DRIFTS results indicate that the low-temperature range of MOF-74-MnCo and MOF-74-Mn is dominated by the E–R mechanism and the high-temperature range is dominated by the L–H mechanism. The entire temperature range of MOF-74-Zn is dominated by the L–H mechanism.
中文翻译:

MOF-74-M(M = Mn,Co,Ni,Zn,MnCo,MnNi和MnZn)用于低温NH 3 -SCR和原位DRIFTS研究反应机理
采用溶剂热法制备了NH 3 -SCR单金属和双金属MOF-74-M(M = Mn,Co,Ni,Zn,MnCo,MnNi和MnZn)催化剂。XRD,BET,SEM和EDS映射测试表明,MOF-74-M催化剂的合成成功,金属元素分布均匀且比表面积大,形态几乎为六边形。将Mn元素添加到单金属催化剂中可以提高活性,这主要是由于存在多种价态的Mn,使其具有优异的氧化还原性能。耐水和硫的催化活性测试表明,加入适量的SO 2后,MOF-74-M的催化活性增加,主要是因为酸性位点的增加。原位DRIFTS结果表明,MOF-74-MnCo和MOF-74-Mn的低温范围受E–R机理支配,而高温范围受L–H机理支配。MOF-74-Zn的整个温度范围受L–H机制控制。
更新日期:2020-10-29
中文翻译:

MOF-74-M(M = Mn,Co,Ni,Zn,MnCo,MnNi和MnZn)用于低温NH 3 -SCR和原位DRIFTS研究反应机理
采用溶剂热法制备了NH 3 -SCR单金属和双金属MOF-74-M(M = Mn,Co,Ni,Zn,MnCo,MnNi和MnZn)催化剂。XRD,BET,SEM和EDS映射测试表明,MOF-74-M催化剂的合成成功,金属元素分布均匀且比表面积大,形态几乎为六边形。将Mn元素添加到单金属催化剂中可以提高活性,这主要是由于存在多种价态的Mn,使其具有优异的氧化还原性能。耐水和硫的催化活性测试表明,加入适量的SO 2后,MOF-74-M的催化活性增加,主要是因为酸性位点的增加。原位DRIFTS结果表明,MOF-74-MnCo和MOF-74-Mn的低温范围受E–R机理支配,而高温范围受L–H机理支配。MOF-74-Zn的整个温度范围受L–H机制控制。































 京公网安备 11010802027423号
京公网安备 11010802027423号