当前位置:
X-MOL 学术
›
J. Clean. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of core-shell α-AlH3@Al(OH)3 nanocomposite with improved low-temperature dehydriding properties by mechanochemical mixing and ionic liquid treatment
Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2020-10-13 , DOI: 10.1016/j.jclepro.2020.124635
Congwen Duan , Zhaohua Su , Yizheng Cao , Lianxi Hu , Dong Fu , Jinlong Ma , Yuling Zhang
Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2020-10-13 , DOI: 10.1016/j.jclepro.2020.124635
Congwen Duan , Zhaohua Su , Yizheng Cao , Lianxi Hu , Dong Fu , Jinlong Ma , Yuling Zhang
|
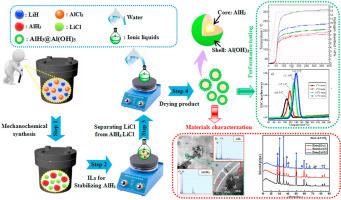
Nano AlH is a promising H storage material, with high gravimetric capacity and dehydriding temperature. However, its instability at ambient conditions and relatively slow dehydriding kinetics hinder its applications. This work reports a simple and environmentally friendly synthesis of a novel core-shell -AlH@Al(OH) nanocomposite with 1.2 nm thick shell. First, -AlH/LiCl nanocomposite was synthesized using a mechanochemical method, during which, AlH was stabilized using imidazolium ionic liquid (IL). After IL application and consequent rinsing with water, -AlH@Al(OH) composite formed. Its dehydriding kinetics was significantly faster (with the activation energy equal to 52.6 kJ/mol) relative to the pure -AlH. At 140 °C, the -AlH@Al(OH) released 10.0 wt% of its original hydrogen content within 1000 s. For comparison, the same amount of hydrogen was released by the commercial -AlH only after 3600 s. The presence of the Al(OH) shell stabilized the -AlH and resulted in absolutely no decomposition of the AlH in the -AlH@Al(OH) composite under ambient conditions for 7 days. Synthesis of this novel core-shell -AlH@Al(OH) nanocomposite is a new and very promising strategy to improve the stability and the hydrogen storage kinetics simultaneously.
中文翻译:

机械化学混合和离子液体处理合成具有改善低温脱氢性能的核壳α-AlH3@Al(OH)3纳米复合材料
纳米AlH是一种很有前途的储氢材料,具有较高的重量容量和脱氢温度。然而,其在环境条件下的不稳定性和相对缓慢的脱氢动力学阻碍了其应用。这项工作报告了一种简单且环保的合成方法,合成了一种具有 1.2 nm 厚壳的新型核壳 -AlH@Al(OH) 纳米复合材料。首先,采用机械化学方法合成了-AlH/LiCl纳米复合材料,在此过程中,使用咪唑鎓离子液体(IL)稳定AlH。在应用 IL 并随后用水冲洗后,形成-AlH@Al(OH) 复合材料。相对于纯-AlH,其脱氢动力学明显更快(活化能等于52.6 kJ/mol)。在 140 °C 时,-AlH@Al(OH) 在 1000 秒内释放了其原始氢含量的 10.0 wt%。为了进行比较,商业-AlH仅在3600秒后才释放出相同量的氢气。 Al(OH)壳的存在稳定了-AlH,并且导致-AlH@Al(OH)复合材料中的AlH在环境条件下7天内绝对没有分解。这种新型核壳-AlH@Al(OH)纳米复合材料的合成是一种新的、非常有前途的策略,可以同时提高稳定性和储氢动力学。
更新日期:2020-10-13
中文翻译:

机械化学混合和离子液体处理合成具有改善低温脱氢性能的核壳α-AlH3@Al(OH)3纳米复合材料
纳米AlH是一种很有前途的储氢材料,具有较高的重量容量和脱氢温度。然而,其在环境条件下的不稳定性和相对缓慢的脱氢动力学阻碍了其应用。这项工作报告了一种简单且环保的合成方法,合成了一种具有 1.2 nm 厚壳的新型核壳 -AlH@Al(OH) 纳米复合材料。首先,采用机械化学方法合成了-AlH/LiCl纳米复合材料,在此过程中,使用咪唑鎓离子液体(IL)稳定AlH。在应用 IL 并随后用水冲洗后,形成-AlH@Al(OH) 复合材料。相对于纯-AlH,其脱氢动力学明显更快(活化能等于52.6 kJ/mol)。在 140 °C 时,-AlH@Al(OH) 在 1000 秒内释放了其原始氢含量的 10.0 wt%。为了进行比较,商业-AlH仅在3600秒后才释放出相同量的氢气。 Al(OH)壳的存在稳定了-AlH,并且导致-AlH@Al(OH)复合材料中的AlH在环境条件下7天内绝对没有分解。这种新型核壳-AlH@Al(OH)纳米复合材料的合成是一种新的、非常有前途的策略,可以同时提高稳定性和储氢动力学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号