Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-10-13 , DOI: 10.1016/j.freeradbiomed.2020.10.008 Parvathy R Suma 1 , Renjini A Padmanabhan 2 , Srinivasa Reddy Telukutla 3 , Rishith Ravindran 4 , Anoop Kumar G Velikkakath 4 , Chaitali D Dekiwadia 5 , Willi Paul 6 , Malini Laloraya 2 , Srinivasa M Srinivasula 4 , Sheshanath V Bhosale 7 , Ramapurath S Jayasree 1
|
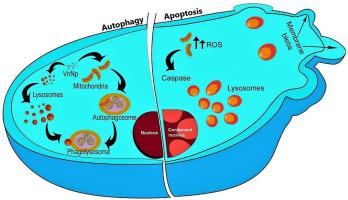
The redox-active transition metals such as copper, iron, chromium, vanadium, and silica are known for its ROS generation via mechanisms such as Haber-Weiss and Fenton-type reactions. Nanoparticles of these metals induce oxidative stress due to acellular factors owing to their small size and more reactive surface area, leading to various cellular responses. The intrinsic enzyme-like activity of nano vanadium has fascinated the scientific community. However, information concerning their cellular uptake and time-dependent induced effects on their cellular organelles and biological activity is lacking. This comprehensive study focuses on understanding the precise molecular interactions of vanadium pentoxide nanoparticles (VnNp) and evaluate their specific “nano” induced effects on MDA-MB-231 cancer cells. Understanding the mechanism behind NP-induced ROS generation could help design a model for selective NP induced toxicity, useful for cancer management. The study demonstrated the intracellular persistence of VnNp and insights into its molecular interactions with various organelles and its overall effects at the cellular level. Where triple-negative breast cancer MDA-MB-231 cells resulted in 59.6% cell death towards 48 h of treatment and the normal fibroblast cells showed only 15.4% cell death, indicating an inherent anticancer property of VnNp. It acts as an initial reactive oxygen species quencher, by serving itself as an antioxidant, while; it was also found to alter the cellular antioxidant system with prolonged incubation. The VnNp accumulated explicitly in the lysosomes and mitochondria and modulated various cellular processes including impaired lysosomal function, mitochondrial damage, and autophagy. At more extended time points, VnNp influenced cell cycle arrest, inhibited cell migration, and potentiated the onset of apoptosis.
Results are indicative of the fact that VnNp selectively induced breast cancer cell death and hence could be developed as a future drug molecule for breast cancer management. This could override the most crucial challenge of chemo-resistance that still remain as the main hurdle to cancer therapy.
中文翻译:

五氧化二钒纳米粒子介导的细胞氧化还原平衡扰动和自噬的凋亡范例
氧化还原活性过渡金属(例如铜,铁,铬,钒和二氧化硅)因通过Haber-Weiss和Fenton型反应等机理而产生ROS而闻名。这些金属的纳米颗粒由于其小尺寸和更大的反应表面积而由于无细胞因素而引起氧化应激,从而导致各种细胞反应。纳米钒的内在酶样活性使科学界着迷。但是,缺乏有关其细胞摄取以及时间依赖性诱导的细胞器和生物活性影响的信息。这项全面的研究着重于了解五氧化二钒纳米颗粒(VnNp)的精确分子相互作用,并评估其对MDA-MB-231癌细胞的特定“纳米”诱导作用。了解NP诱导的ROS产生的机制可能有助于设计选择性NP诱导的毒性模型,对癌症治疗很有用。这项研究证明了VnNp在细胞内的持久性,并深入了解了其与各种细胞器的分子相互作用以及在细胞水平上的总体作用。三阴性乳腺癌MDA-MB-231细胞在治疗48小时后导致59.6%的细胞死亡,而正常的成纤维细胞仅显示15.4%的细胞死亡,表明VnNp具有固有的抗癌特性。它通过自身作为抗氧化剂而充当初始的活性氧猝灭剂。还发现长时间孵育会改变细胞的抗氧化剂系统。VnNp在溶酶体和线粒体中明显积累,并调节了各种细胞过程,包括溶酶体功能受损,线粒体损伤和自噬。在更长的时间点,VnNp影响细胞周期停滞,抑制细胞迁移,并增强凋亡的发生。
结果表明以下事实:VnNp选择性诱导乳腺癌细胞死亡,因此可以作为乳腺癌治疗的未来药物分子开发。这可以克服化学抗性最关键的挑战,而化学抗性仍然是癌症治疗的主要障碍。















































 京公网安备 11010802027423号
京公网安备 11010802027423号