当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of benzamide derivatives with thiourea‐substituted benzenesulfonamides as carbonic anhydrase inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-10-12 , DOI: 10.1002/ardp.202000230 Mehtap Tugrak 1 , Halise Inci Gul 1 , Yeliz Demir 2 , Ilhami Gulcin 3
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-10-12 , DOI: 10.1002/ardp.202000230 Mehtap Tugrak 1 , Halise Inci Gul 1 , Yeliz Demir 2 , Ilhami Gulcin 3
Affiliation
|
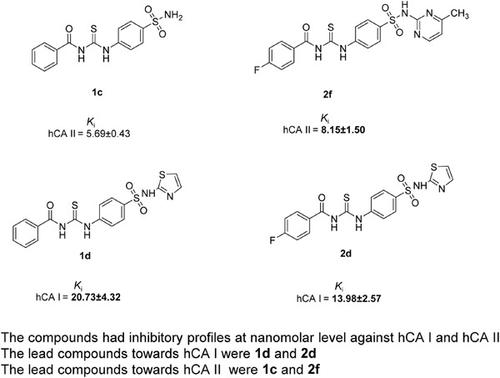
The novel compounds with the chemical structure of N-({4-[N'-(substituted)sulfamoyl]phenyl}carbamothioyl)benzamide (1a-g) and 4-fluoro-N-({4-[N'-(substituted)sulfamoyl]phenyl}carbamothioyl)benzamide (2a-g) were synthesized as potent and selective human carbonic anhydrase (hCA) I and hCA II candidate inhibitors. The aryl part was changed to sulfacetamide, sulfaguanidine, sulfanilamide, sulfathiazole, sulfadiazine, sulfamerazine, and sulfametazine. The Ki values of compounds 1a-g were in the range of 20.73 ± 4.32 to 59.55 ± 13.07 nM (hCA I) and 5.69 ± 0.43 to 44.81 ± 1.08 nM (hCA II), whereas the Ki values of compounds 2a-g were in the range of 13.98 ± 2.57 to 75.74 ± 13.51 nM (hCA I) and 8.15 ± 1.5 to 49.86 ± 6.18 nM (hCA II). Comparing the Ki values of the final compounds and acetazolamide, compound 1c with the sulfanilamide moiety (Ki = 5.69 ± 0.43 nM, 8.8 times) and 2f with the sulfamerazine moiety (Ki = 8.15 ± 1.5 nM, 6.2 times) demonstrated promising and selective inhibitory effects against the hCA II isoenzyme, the main target protein in glaucoma. Furthermore, compounds 1d (Ki = 20.73 ± 4.32, 4 times) and 2d (Ki = 13.98 ± 2.57, 5.9 times), which have the sulfathiazole moiety, were found as potent hCA I inhibitors. Compounds 1c and 2f can be considered as the lead compounds determined in the present study, which can be investigated further to alleviate glaucoma symptoms.
中文翻译:

硫脲取代苯磺酰胺作为碳酸酐酶抑制剂合成苯甲酰胺衍生物
化学结构为N-({4-[N'-(取代)氨磺酰基]苯基}氨基甲硫酰基)苯甲酰胺(1a-g)和4-氟-N-({4-[N'-(取代) )氨磺酰基]苯基}氨基甲硫酰基)苯甲酰胺 (2a-g) 被合成为有效和选择性的人碳酸酐酶 (hCA) I 和 hCA II 候选抑制剂。芳基部分变为磺胺醋酰胺、磺胺胍、磺胺、磺胺噻唑、磺胺嘧啶、磺胺嘧啶和磺胺二甲嘧啶。化合物 1a-g 的 Ki 值在 20.73 ± 4.32 至 59.55 ± 13.07 nM (hCA I) 和 5.69 ± 0.43 至 44.81 ± 1.08 nM (hCA II) 的范围内,而化合物 2a-g 的 Ki 值在13.98 ± 2.57 至 75.74 ± 13.51 nM (hCA I) 和 8.15 ± 1.5 至 49.86 ± 6.18 nM (hCA II) 的范围。比较最终化合物和乙酰唑胺的 Ki 值,化合物 1c 与磺胺部分 (Ki = 5. 69 ± 0.43 nM,8.8 倍)和 2f 与磺胺嘧啶部分(Ki = 8.15 ± 1.5 nM,6.2 倍)显示出对 hCA II 同工酶(青光眼的主要靶蛋白)的有希望和选择性的抑制作用。此外,发现具有磺胺噻唑部分的化合物 1d(Ki = 20.73 ± 4.32,4 倍)和 2d(Ki = 13.98 ± 2.57,5.9 倍)是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。具有磺胺噻唑部分的 HCA I 抑制剂被发现是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。具有磺胺噻唑部分的 HCA I 抑制剂被发现是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。
更新日期:2020-10-12
中文翻译:

硫脲取代苯磺酰胺作为碳酸酐酶抑制剂合成苯甲酰胺衍生物
化学结构为N-({4-[N'-(取代)氨磺酰基]苯基}氨基甲硫酰基)苯甲酰胺(1a-g)和4-氟-N-({4-[N'-(取代) )氨磺酰基]苯基}氨基甲硫酰基)苯甲酰胺 (2a-g) 被合成为有效和选择性的人碳酸酐酶 (hCA) I 和 hCA II 候选抑制剂。芳基部分变为磺胺醋酰胺、磺胺胍、磺胺、磺胺噻唑、磺胺嘧啶、磺胺嘧啶和磺胺二甲嘧啶。化合物 1a-g 的 Ki 值在 20.73 ± 4.32 至 59.55 ± 13.07 nM (hCA I) 和 5.69 ± 0.43 至 44.81 ± 1.08 nM (hCA II) 的范围内,而化合物 2a-g 的 Ki 值在13.98 ± 2.57 至 75.74 ± 13.51 nM (hCA I) 和 8.15 ± 1.5 至 49.86 ± 6.18 nM (hCA II) 的范围。比较最终化合物和乙酰唑胺的 Ki 值,化合物 1c 与磺胺部分 (Ki = 5. 69 ± 0.43 nM,8.8 倍)和 2f 与磺胺嘧啶部分(Ki = 8.15 ± 1.5 nM,6.2 倍)显示出对 hCA II 同工酶(青光眼的主要靶蛋白)的有希望和选择性的抑制作用。此外,发现具有磺胺噻唑部分的化合物 1d(Ki = 20.73 ± 4.32,4 倍)和 2d(Ki = 13.98 ± 2.57,5.9 倍)是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。具有磺胺噻唑部分的 HCA I 抑制剂被发现是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。具有磺胺噻唑部分的 HCA I 抑制剂被发现是有效的 hCA I 抑制剂。化合物1c和2f可以被认为是本研究中确定的先导化合物,可以进一步研究以减轻青光眼症状。





























 京公网安备 11010802027423号
京公网安备 11010802027423号