当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards Naked Zinc(II) in the Condensed Phase: A Highly Lewis Acidic ZnII Dication Stabilized by Weakly Coordinating Carborate Anions
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-09 , DOI: 10.1002/anie.202012287 Nicolas Adet 1 , David Specklin 1 , Christophe Gourlaouen 1 , Thibault Damiens 1 , Béatrice Jacques 1 , Rudolf J. Wehmschulte 2 , Samuel Dagorne 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-09 , DOI: 10.1002/anie.202012287 Nicolas Adet 1 , David Specklin 1 , Christophe Gourlaouen 1 , Thibault Damiens 1 , Béatrice Jacques 1 , Rudolf J. Wehmschulte 2 , Samuel Dagorne 1
Affiliation
|
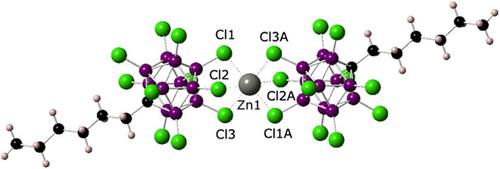
The employment of the hexyl‐substituted anion [HexCB11Cl11]− allowed the synthesis of a ZnII species, Zn[HexCB11Cl11]2, 3, in which the Zn2+ cation is only weakly coordinated to two carborate counterions and that is soluble in low polarity organic solvents such as bromobenzene. DOSY NMR studies show the facile displacement of at least one of the counterions, and this near nakedness of the cation results in high catalytic activity in the hydrosilylation of 1‐hexene and 1‐methyl‐1cyclohexene. Fluoride ion affinity (FIA) calculations reveal a solution Lewis acidity of 3 (FIA=262.1 kJ mol−1) that is higher than that of the landmark Lewis acid B(C6F5)3 (FIA=220.5 kJ mol−1). This high Lewis acidity leads to a high activity in catalytic CO2 and Ph2CO reduction by Et3SiH and hydrogenation of 1,1‐diphenylethylene using 1,4‐cyclohexadiene as the hydrogen source. Compound 3 was characterized by multinuclear NMR spectroscopy, mass spectrometry, single crystal X‐ray diffraction, and DFT studies.
中文翻译:

缩合相中的裸锌(II):弱配位的硼酸根阴离子稳定的高路易斯酸性ZnII阳离子
己基-取代的阴离子的就业[HexCB 11氯11 ] -允许将Zn的合成II物种,锌[HexCB 11氯11 ] 2,3,其中锌2+阳离子仅弱配位于两个碳硼烷抗衡离子易溶于低极性有机溶剂,如溴苯。DOSY NMR研究表明,至少一种抗衡离子很容易置换,而阳离子的这种近乎裸露的状态导致1-己烯和1-甲基-1环己烯的氢化硅烷化反应具有很高的催化活性。氟离子亲和力(FIA)计算表明溶液的路易斯酸度为3(FIA = 262.1kJ mol -1)高于地标路易斯酸B(C 6 F 5)3的FIA = 222.1kJ mol -1。这种高的路易斯酸度导致Et 3 SiH催化的CO 2和Ph 2 CO还原以及使用1,4-环己二烯作为氢源的1,1-二苯乙烯加氢的高活性。通过多核NMR光谱,质谱,单晶X射线衍射和DFT研究来表征化合物3。
更新日期:2020-10-09
中文翻译:

缩合相中的裸锌(II):弱配位的硼酸根阴离子稳定的高路易斯酸性ZnII阳离子
己基-取代的阴离子的就业[HexCB 11氯11 ] -允许将Zn的合成II物种,锌[HexCB 11氯11 ] 2,3,其中锌2+阳离子仅弱配位于两个碳硼烷抗衡离子易溶于低极性有机溶剂,如溴苯。DOSY NMR研究表明,至少一种抗衡离子很容易置换,而阳离子的这种近乎裸露的状态导致1-己烯和1-甲基-1环己烯的氢化硅烷化反应具有很高的催化活性。氟离子亲和力(FIA)计算表明溶液的路易斯酸度为3(FIA = 262.1kJ mol -1)高于地标路易斯酸B(C 6 F 5)3的FIA = 222.1kJ mol -1。这种高的路易斯酸度导致Et 3 SiH催化的CO 2和Ph 2 CO还原以及使用1,4-环己二烯作为氢源的1,1-二苯乙烯加氢的高活性。通过多核NMR光谱,质谱,单晶X射线衍射和DFT研究来表征化合物3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号