当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and antimicrobial activity of new 2‐piperazin‐1‐yl‐ N ‐1,3‐thiazol‐2‐ylacetamides of cyclopenta[ c ]pyridines and pyrano[3,4‐ c ]pyridines
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-10-07 , DOI: 10.1002/ardp.202000208 Samvel Sirakanyan 1 , Victor Kartsev 2 , Domenico Spinelli 3 , Athina Geronikaki 4 , Anthi Petrou 4 , Marija Ivanov 5 , Jasmina Glamoclija 5 , Marina Sokovic 5 , Elmira Hakobyan 1 , Anush Hovakimyan 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-10-07 , DOI: 10.1002/ardp.202000208 Samvel Sirakanyan 1 , Victor Kartsev 2 , Domenico Spinelli 3 , Athina Geronikaki 4 , Anthi Petrou 4 , Marija Ivanov 5 , Jasmina Glamoclija 5 , Marina Sokovic 5 , Elmira Hakobyan 1 , Anush Hovakimyan 1
Affiliation
|
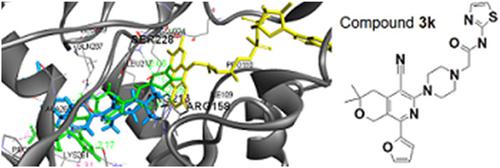
In this study, we report the synthesis and antimicrobial activity of some new disubstituted piperazines. Thus, 3‐chlorocyclopenta[c]pyridines and 6‐chloropyrano[3,4‐c]pyridine 1 under mild reaction conditions with piperazine gave the 3(6)‐piperazine‐substituted cyclopenta[c]pyridines and pyrano[3,4‐c]pyridine 2. Furthermore, the latter, by alkylation with 2‐chloro‐N‐1,3‐thiazol‐2‐ylacetamide, led to the formation of the target compounds. The evaluation of the antibacterial activity revealed that 3k was the most potent compound. The most sensitive bacterium was found to be Listeria monocytogenes, whereas Staphylococcus aureus was the most resistant one. Three compounds, 3d, 3g, and 3k, were tested also against the following resistant strains: methicillin‐resistant S. aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. All three compounds appeared to be more potent than ampicillin against MRSA. Moreover, compound 3d showed a better activity than the reference drug ampicillin against P. aeruginosa, whereas 3g was more efficient against E. coli. The best antifungal activity was observed again for compound 3k. The most resistant fungi appeared to be Aspergillus fumigatus, whereas Trichoderma viride seemed the most sensitive one toward the compounds tested. Molecular docking studies on E. coli MurB, as well as on Candida albicans CYP51 and dihydrofolate reductase, were used for the prediction of the mechanisms of the antibacterial and antifungal activities, confirming the experimental results.
中文翻译:

环戊[c]吡啶和吡喃并[3,4-c]吡啶的新型2-哌嗪-1-基-N-1,3-噻唑-2-基乙酰胺的合成及抗菌活性
在这项研究中,我们报告了一些新的双取代哌嗪的合成和抗菌活性。因此,3-氯环戊[c]吡啶和6-氯吡喃[3,4-c]吡啶1在温和的条件下与哌嗪反应得到3(6)-哌嗪取代的环戊[c]吡啶和吡喃[3,4- c]吡啶2。此外,后者通过与2-氯-N-1,3-噻唑-2-基乙酰胺的烷基化,导致目标化合物的形成。抗菌活性的评估表明,3k 是最有效的化合物。发现最敏感的细菌是单核细胞增生李斯特菌,而金黄色葡萄球菌是最耐药的细菌。还针对以下耐药菌株测试了三种化合物 3d、3g 和 3k:耐甲氧西林金黄色葡萄球菌 (MRSA)、大肠杆菌和铜绿假单胞菌。所有三种化合物似乎都比氨苄西林更有效地对抗 MRSA。此外,化合物 3d 对铜绿假单胞菌表现出比参考药物氨苄青霉素更好的活性,而 3g 对大肠杆菌的活性更高。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。而 3g 对大肠杆菌更有效。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。而 3g 对大肠杆菌更有效。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。
更新日期:2020-10-07
中文翻译:

环戊[c]吡啶和吡喃并[3,4-c]吡啶的新型2-哌嗪-1-基-N-1,3-噻唑-2-基乙酰胺的合成及抗菌活性
在这项研究中,我们报告了一些新的双取代哌嗪的合成和抗菌活性。因此,3-氯环戊[c]吡啶和6-氯吡喃[3,4-c]吡啶1在温和的条件下与哌嗪反应得到3(6)-哌嗪取代的环戊[c]吡啶和吡喃[3,4- c]吡啶2。此外,后者通过与2-氯-N-1,3-噻唑-2-基乙酰胺的烷基化,导致目标化合物的形成。抗菌活性的评估表明,3k 是最有效的化合物。发现最敏感的细菌是单核细胞增生李斯特菌,而金黄色葡萄球菌是最耐药的细菌。还针对以下耐药菌株测试了三种化合物 3d、3g 和 3k:耐甲氧西林金黄色葡萄球菌 (MRSA)、大肠杆菌和铜绿假单胞菌。所有三种化合物似乎都比氨苄西林更有效地对抗 MRSA。此外,化合物 3d 对铜绿假单胞菌表现出比参考药物氨苄青霉素更好的活性,而 3g 对大肠杆菌的活性更高。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。而 3g 对大肠杆菌更有效。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。而 3g 对大肠杆菌更有效。对于化合物3k,再次观察到最好的抗真菌活性。最具抗性的真菌似乎是烟曲霉,而绿色木霉似乎对测试的化合物最敏感。对大肠杆菌 MurB 以及白色念珠菌 CYP51 和二氢叶酸还原酶的分子对接研究用于预测抗菌和抗真菌活性的机制,证实了实验结果。






























 京公网安备 11010802027423号
京公网安备 11010802027423号