当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light‐Promoted Copper‐Catalyzed Enantioselective Alkylation of Azoles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202009323 Chen Li 1 , Bin Chen 1 , Xiaodong Ma 2 , Xueling Mo 1 , Guozhu Zhang 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202009323 Chen Li 1 , Bin Chen 1 , Xiaodong Ma 2 , Xueling Mo 1 , Guozhu Zhang 3
Affiliation
|
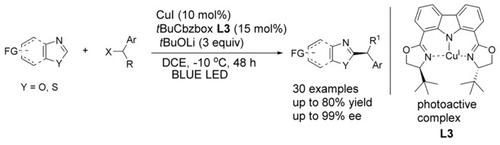
A catalytic asymmetric alkylation of azoles with secondary 1‐arylalkyl bromides through direct C−H functionalization is reported. Under blue‐light photoexcitation, a copper(I)/carbazole‐based bisoxazoline (CbzBox) catalytic system exhibits good reactivity and high stereoselectivity, thus offering an efficient strategy for the construction of chiral alkyl azoles. These reactions proceed at low temperature and are compatible with a wide range of azoles.
中文翻译:

光促进的铜催化的对氮苯的对映选择性烷基化
据报道,通过直接的C H官能化,仲仲1-芳基烷基溴催化了唑类的催化不对称烷基化。在蓝光光激发下,基于铜(I)/咔唑的双恶唑啉(CbzBox)催化体系表现出良好的反应性和高的立体选择性,从而为构建手性烷基唑提供了有效的策略。这些反应在低温下进行,并与各种唑类相容。
更新日期:2020-10-07
中文翻译:

光促进的铜催化的对氮苯的对映选择性烷基化
据报道,通过直接的C H官能化,仲仲1-芳基烷基溴催化了唑类的催化不对称烷基化。在蓝光光激发下,基于铜(I)/咔唑的双恶唑啉(CbzBox)催化体系表现出良好的反应性和高的立体选择性,从而为构建手性烷基唑提供了有效的策略。这些反应在低温下进行,并与各种唑类相容。































 京公网安备 11010802027423号
京公网安备 11010802027423号