Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Bromoanthraquinone as a highly efficient photocatalyst for the oxidation of sec-aromatic alcohols: experimental and DFT study
RSC Advances ( IF 3.9 ) Pub Date : 2020-10-7 , DOI: 10.1039/d0ra06414a Shengfu Liao 1, 2 , Jianguo Liu 1 , Long Yan 1 , Qiying Liu 1, 2 , Guanghui Chen 3 , Longlong Ma 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2020-10-7 , DOI: 10.1039/d0ra06414a Shengfu Liao 1, 2 , Jianguo Liu 1 , Long Yan 1 , Qiying Liu 1, 2 , Guanghui Chen 3 , Longlong Ma 1, 2
Affiliation
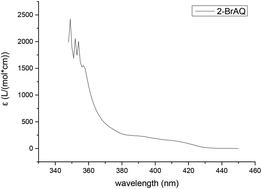
|
Anthraquinones are recognized as high efficiency photocatalysts which can perform various redox reactions in aqueous or organic phases. We have experimentally proven that 2-BrAQ can undergo hydrogen transfer with an alpha-aromatic alcohol under light conditions, thereby efficiently oxidizing the aromatic alcohol to the corresponding product. The yield of 1-phenethanol to acetophenone can reach more than 96%. In subsequent catalyst screening experiments, it was found that the electronegativity of the substituent at the 2 position of the anthraquinone ring and the acidity of the solvent affect the photocatalytic activity of anthraquinones. After using various aromatic alcohol substrates, 2-BrAQ showed good conversion and selectivity for most aromatic alcohols, but showed C–C bond cleavage and low selectivity with non-α-position aromatic alcohols. In order to explore the mechanism of the redox reaction of 2-BrAQ in acetonitrile solution, the corresponding free radical reaction pathway was proposed and verified by density functional theory (DFT). Focusing on calculations for 2-BrAQ during the reaction and the first-step hydrogen transfer reaction between the 2-BrAQ triplet molecule and the 1-phenylethanol molecule, we recognized the changes that occurred during the reaction and thus have a deeper understanding of the redox reaction of anthraquinone compounds in organic systems.
中文翻译:

2-溴蒽醌作为仲芳香醇氧化的高效光催化剂:实验和DFT研究
蒽醌被认为是高效光催化剂,可以在水相或有机相中进行各种氧化还原反应。我们通过实验证明2-BrAQ可以在光照条件下与α-芳香醇进行氢转移,从而有效地将芳香醇氧化成相应的产物。 1-苯乙醇制苯乙酮的收率可达96%以上。在后续的催化剂筛选实验中,发现蒽醌环2位取代基的电负性和溶剂的酸性影响蒽醌类化合物的光催化活性。使用各种芳香醇底物后,2-BrAQ对大多数芳香醇表现出良好的转化率和选择性,但对非α位芳香醇表现出C-C键断裂和低选择性。为了探索2-BrAQ在乙腈溶液中的氧化还原反应机理,提出了相应的自由基反应路径,并通过密度泛函理论(DFT)进行了验证。重点关注2-BrAQ反应过程中的计算以及2-BrAQ三重态分子与1-苯基乙醇分子之间的第一步氢转移反应,我们认识到反应过程中发生的变化,从而对氧化还原有了更深入的了解有机体系中蒽醌化合物的反应。
更新日期:2020-10-07
中文翻译:

2-溴蒽醌作为仲芳香醇氧化的高效光催化剂:实验和DFT研究
蒽醌被认为是高效光催化剂,可以在水相或有机相中进行各种氧化还原反应。我们通过实验证明2-BrAQ可以在光照条件下与α-芳香醇进行氢转移,从而有效地将芳香醇氧化成相应的产物。 1-苯乙醇制苯乙酮的收率可达96%以上。在后续的催化剂筛选实验中,发现蒽醌环2位取代基的电负性和溶剂的酸性影响蒽醌类化合物的光催化活性。使用各种芳香醇底物后,2-BrAQ对大多数芳香醇表现出良好的转化率和选择性,但对非α位芳香醇表现出C-C键断裂和低选择性。为了探索2-BrAQ在乙腈溶液中的氧化还原反应机理,提出了相应的自由基反应路径,并通过密度泛函理论(DFT)进行了验证。重点关注2-BrAQ反应过程中的计算以及2-BrAQ三重态分子与1-苯基乙醇分子之间的第一步氢转移反应,我们认识到反应过程中发生的变化,从而对氧化还原有了更深入的了解有机体系中蒽醌化合物的反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号