当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
g‐C3N4 Derivative Artificial Organic/Inorganic Composite Solid Electrolyte Interphase Layer for Stable Lithium Metal Anode
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-10-07 , DOI: 10.1002/aenm.202002647 Shufen Ye 1 , Lifeng Wang 1 , Fanfan Liu 1 , Pengcheng Shi 1 , Haiyun Wang 1 , Xiaojun Wu 1 , Yan Yu 1, 2
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-10-07 , DOI: 10.1002/aenm.202002647 Shufen Ye 1 , Lifeng Wang 1 , Fanfan Liu 1 , Pengcheng Shi 1 , Haiyun Wang 1 , Xiaojun Wu 1 , Yan Yu 1, 2
Affiliation
|
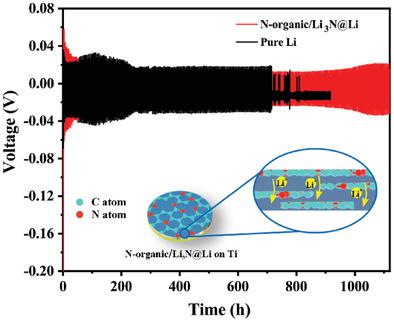
Lithium metal anodes are one of the most promising anodes in “next‐generation” rechargeable batteries. However, continuous dendrite growth and interface instability of the anode have prevented practical applications. Constructing an artificial solid electrolyte interphase (SEI) is an effective way to solve these issues. Herein, an artificial organic/inorganic SEI layer (denoted as N‐organic/Li3N) is designed, consisting of Li2CN2 and Li3N phases, to achieve stable cycling of Li metal electrodes. Density functional theory (DFT) results reveal that the N‐organic/Li3N layer with a high Li ionic conductivity can effectively facilitate the transport of Li ions across the electrode surface and lead to uniform Li ionic flux on Li electrodes via strong interactions between Li ions and N‐organic groups, resulting in dendrite‐free Li stripping/plating. The N‐organic/Li3N‐coated Li (denoted as N‐organic/Li3N@Li) anode delivers stable long‐term cycling performance over 1100 h with a fixed areal capacity of 2 mAh cm−2 under 1 mA cm−2. A full battery assembled with a LiNi0.6Co0.2Mn0.2O2 (NCM622) cathode displays better long‐term cycle performance when the N‐organic/Li3N@Li composite anode is applied. The advantages of the organic/inorganic artificial SEI provide important insights into the design principles of SEI for lithium metal anodes.
中文翻译:

g-C3N4稳定的锂金属阳极人造有机/无机复合固体电解质相间层
锂金属阳极是“下一代”可充电电池中最有希望的阳极之一。然而,阳极的连续枝晶生长和界面不稳定性阻碍了实际应用。构建人工固体电解质中间相(SEI)是解决这些问题的有效方法。在此,设计了由Li 2 CN 2和Li 3 N相组成的人造有机/无机SEI层(表示为N-organic / Li 3 N),以实现Li金属电极的稳定循环。密度泛函理论(DFT)结果显示N-organic / Li 3具有高Li离子电导率的N层可以有效地促进Li离子在电极表面上的传输,并通过Li离子与N有机基团之间的强相互作用在Li电极上导致均匀的Li离子通量,从而导致无枝晶的Li剥离/电镀。N-有机/ Li 3 N涂覆的Li(表示为N-有机/ Li 3 N @ Li)阳极在1100 h内提供稳定的长期循环性能,在1 mA cm下的固定面积容量为2 mAh cm -2 −2。当使用N-有机物/ Li 3时,与LiNi 0.6 Co 0.2 Mn 0.2 O 2(NCM622)阴极组装的完整电池具有更好的长期循环性能。使用N @ Li复合阳极。有机/无机人工SEI的优势为锂金属阳极SEI的设计原理提供了重要见识。
更新日期:2020-11-25
中文翻译:

g-C3N4稳定的锂金属阳极人造有机/无机复合固体电解质相间层
锂金属阳极是“下一代”可充电电池中最有希望的阳极之一。然而,阳极的连续枝晶生长和界面不稳定性阻碍了实际应用。构建人工固体电解质中间相(SEI)是解决这些问题的有效方法。在此,设计了由Li 2 CN 2和Li 3 N相组成的人造有机/无机SEI层(表示为N-organic / Li 3 N),以实现Li金属电极的稳定循环。密度泛函理论(DFT)结果显示N-organic / Li 3具有高Li离子电导率的N层可以有效地促进Li离子在电极表面上的传输,并通过Li离子与N有机基团之间的强相互作用在Li电极上导致均匀的Li离子通量,从而导致无枝晶的Li剥离/电镀。N-有机/ Li 3 N涂覆的Li(表示为N-有机/ Li 3 N @ Li)阳极在1100 h内提供稳定的长期循环性能,在1 mA cm下的固定面积容量为2 mAh cm -2 −2。当使用N-有机物/ Li 3时,与LiNi 0.6 Co 0.2 Mn 0.2 O 2(NCM622)阴极组装的完整电池具有更好的长期循环性能。使用N @ Li复合阳极。有机/无机人工SEI的优势为锂金属阳极SEI的设计原理提供了重要见识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号