当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of α-Chiral Propargylic Silanes by Copper-Catalyzed 1,4-Selective Addition of Silicon Nucleophiles to Enyne-Type α,β,γ,δ-Unsaturated Acceptors
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-06 , DOI: 10.1021/acs.orglett.0c03046
Wenbin Mao 1 , Martin Oestreich 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-06 , DOI: 10.1021/acs.orglett.0c03046
Wenbin Mao 1 , Martin Oestreich 1
Affiliation
|
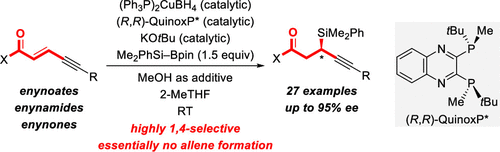
A copper-catalyzed deconjugative addition of silicon nucleophiles to a broad range of enyne-type α,β,γ,δ-unsaturated acceptors with high enantiocontrol is reported. The method is 1,4-selective with hardly any formation of the 1,6-adduct. The double-bond geometry is shown to be critical for achieving this chemoselectivity: exclusive 1,4-addition for E and predominant 1,6-addition for Z. By this, E-configured enynoates, enynamides, and enynones have been converted to the corresponding α-chiral propargylic silanes with excellent enantiomeric excesses.
中文翻译:

铜亲核硅与烯炔型α,β,γ,δ-不饱和受体的1,4-选择性加成反应,对映体选择性合成α-手性炔丙基硅烷
据报道,铜亲核试剂将硅亲核试剂铜键解离共轭添加到具有高对映体控制作用的各种烯炔型α,β,γ,δ不饱和受体上。该方法是1,4-选择性的,几乎没有形成1,6-加合物。已显示双键几何结构对于实现这种化学选择性至关重要:E的排他1,4-加成和Z的主要1,6-加成。由此,E-构型的烯酸酯,烯酰胺和烯酮已被转化为具有优异对映体过量的相应的α-手性炔丙基硅烷。
更新日期:2020-10-17
中文翻译:

铜亲核硅与烯炔型α,β,γ,δ-不饱和受体的1,4-选择性加成反应,对映体选择性合成α-手性炔丙基硅烷
据报道,铜亲核试剂将硅亲核试剂铜键解离共轭添加到具有高对映体控制作用的各种烯炔型α,β,γ,δ不饱和受体上。该方法是1,4-选择性的,几乎没有形成1,6-加合物。已显示双键几何结构对于实现这种化学选择性至关重要:E的排他1,4-加成和Z的主要1,6-加成。由此,E-构型的烯酸酯,烯酰胺和烯酮已被转化为具有优异对映体过量的相应的α-手性炔丙基硅烷。

































 京公网安备 11010802027423号
京公网安备 11010802027423号