当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Streamlined Catalytic Enantioselective Synthesis of α-Substituted β,γ-Unsaturated Ketones and Either of the Corresponding Tertiary Homoallylic Alcohol Diastereomers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-05 , DOI: 10.1021/jacs.0c08732
Juan Del Pozo 1 , Shaochen Zhang 1 , Filippo Romiti 1, 2 , Shibo Xu 1 , Ryan P Conger 1 , Amir H Hoveyda 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-05 , DOI: 10.1021/jacs.0c08732
Juan Del Pozo 1 , Shaochen Zhang 1 , Filippo Romiti 1, 2 , Shibo Xu 1 , Ryan P Conger 1 , Amir H Hoveyda 1, 2
Affiliation
|
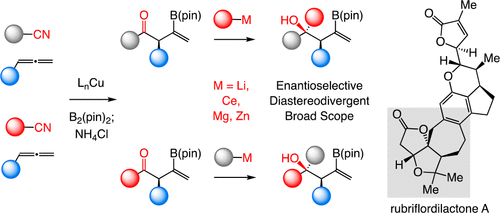
A widely applicable, practical, and scalable strategy for efficient and enantioselective synthesis of β,γ-unsaturated ketones that contain an α-stereogenic center is disclosed. Accordingly, aryl, heteroaryl, alkynyl, alkenyl, allyl, or alkyl ketones that contain an α-stereogenic carbon with an alkyl, an aryl, a benzyloxy, or a siloxy moiety can be generated from readily available starting materials and by the use of commercially available chiral ligands in 52-96% yield and 93:7 to >99:1 enantiomeric ratio. To develop the new method, conditions were identified so that high enantioselectivity would be attained and the resulting α-substituted NH-ketimines, wherein there is strong C═N → B(pin) coordination, would not epimerize before conversion to the derived ketone by hydrolysis. It is demonstrated that the ketone products can be converted to an assortment of homoallylic tertiary alcohols in 70-96% yield and 92:8 to >98:2 dr-in either diastereomeric form-by reactions with alkyl-, aryl-, heteroaryl-, allyl-, vinyl-, alkynyl-, or propargyl-metal reagents. The utility of the approach is highlighted through transformations that furnish other desirable derivatives and a concise synthesis route affording more than a gram of a major fragment of anti-HIV agents rubriflordilactones A and B and a specific stereoisomeric analogue.
中文翻译:

α-取代的 β,γ-不饱和酮和相应的叔高烯丙醇非对映体的简化催化对映选择性合成
公开了一种广泛适用、实用且可扩展的策略,用于有效和对映选择性合成含有 α-立体中心的 β,γ-不饱和酮。因此,包含具有烷基、芳基、苄氧基或甲硅烷氧基部分的α-立构碳的芳基、杂芳基、炔基、烯基、烯丙基或烷基酮可以由易于获得的起始材料并通过使用市售原料来产生。可用手性配体的产率为 52-96%,对映体比例为 93:7 至 >99:1。为了开发新方法,确定了获得高对映选择性的条件,并且所得的 α-取代的 NH-酮亚胺(其中存在强 C=N → B(pin) 配位)在转化为衍生酮之前不会差向异构化水解。结果表明,通过与烷基-、芳基-、杂芳基-反应,酮产物可以以任何非对映体形式转化为各种高烯丙基叔醇,收率70-96%,比例为92:8至>98:2。 、烯丙基-、乙烯基-、炔基-或炔丙基-金属试剂。该方法的实用性通过提供其他所需衍生物的转化和提供超过一克的抗HIV药物rubriflordilactones A和B的主要片段以及特定的立体异构类似物的简明合成路线而得到强调。
更新日期:2020-10-05
中文翻译:

α-取代的 β,γ-不饱和酮和相应的叔高烯丙醇非对映体的简化催化对映选择性合成
公开了一种广泛适用、实用且可扩展的策略,用于有效和对映选择性合成含有 α-立体中心的 β,γ-不饱和酮。因此,包含具有烷基、芳基、苄氧基或甲硅烷氧基部分的α-立构碳的芳基、杂芳基、炔基、烯基、烯丙基或烷基酮可以由易于获得的起始材料并通过使用市售原料来产生。可用手性配体的产率为 52-96%,对映体比例为 93:7 至 >99:1。为了开发新方法,确定了获得高对映选择性的条件,并且所得的 α-取代的 NH-酮亚胺(其中存在强 C=N → B(pin) 配位)在转化为衍生酮之前不会差向异构化水解。结果表明,通过与烷基-、芳基-、杂芳基-反应,酮产物可以以任何非对映体形式转化为各种高烯丙基叔醇,收率70-96%,比例为92:8至>98:2。 、烯丙基-、乙烯基-、炔基-或炔丙基-金属试剂。该方法的实用性通过提供其他所需衍生物的转化和提供超过一克的抗HIV药物rubriflordilactones A和B的主要片段以及特定的立体异构类似物的简明合成路线而得到强调。

































 京公网安备 11010802027423号
京公网安备 11010802027423号