当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Catalyzed Enantioselective Alkylarylation of Vinylarenes Enabled by Chiral Binaphthyl–BOX Hybrid Ligands
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-05 , DOI: 10.1021/jacs.0c09008 Shunya Sakurai 1 , Akira Matsumoto 2 , Taichi Kano 1 , Keiji Maruoka 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-05 , DOI: 10.1021/jacs.0c09008 Shunya Sakurai 1 , Akira Matsumoto 2 , Taichi Kano 1 , Keiji Maruoka 1, 2, 3
Affiliation
|
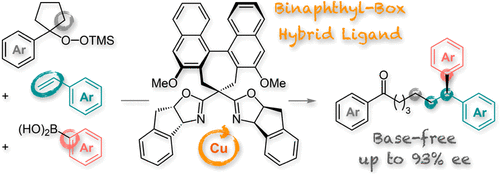
Transition-metal-catalyzed radical relay coupling reactions have recently emerged as one of the most powerful methods to achieve difunctionalization of olefins. However, there has been limited success in applying this method to asymmetric catalysis using an effective chiral ligand. Herein we report the Cu-catalyzed enantioselective alkylarylation of vinylarenes using alkylsilyl peroxides as alkyl radical sources. This reaction proceeds under practical reaction conditions and affords chiral 1,1-diarylalkane structures that are found in a variety of bioactive molecules. Notably, a highly enantioselective reaction was accomplished by combining chiral bis(oxazoline) ligands with chiral binaphthyl scaffolds.
中文翻译:

由手性联萘-BOX杂化配体实现的乙烯基芳烃的铜催化对映选择性烷基芳基化
过渡金属催化的自由基中继偶联反应最近已成为实现烯烃双官能化的最有效方法之一。然而,在使用有效手性配体将这种方法应用于不对称催化方面的成功有限。在此,我们报告了使用烷基甲硅烷基过氧化物作为烷基自由基源的 Cu 催化的乙烯基芳烃的对映选择性烷基芳基化。该反应在实际反应条件下进行,并提供在各种生物活性分子中发现的手性 1,1-二芳基烷烃结构。值得注意的是,高度对映选择性反应是通过将手性双(恶唑啉)配体与手性联萘支架结合来实现的。
更新日期:2020-10-05
中文翻译:

由手性联萘-BOX杂化配体实现的乙烯基芳烃的铜催化对映选择性烷基芳基化
过渡金属催化的自由基中继偶联反应最近已成为实现烯烃双官能化的最有效方法之一。然而,在使用有效手性配体将这种方法应用于不对称催化方面的成功有限。在此,我们报告了使用烷基甲硅烷基过氧化物作为烷基自由基源的 Cu 催化的乙烯基芳烃的对映选择性烷基芳基化。该反应在实际反应条件下进行,并提供在各种生物活性分子中发现的手性 1,1-二芳基烷烃结构。值得注意的是,高度对映选择性反应是通过将手性双(恶唑啉)配体与手性联萘支架结合来实现的。































 京公网安备 11010802027423号
京公网安备 11010802027423号