Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-04 , DOI: 10.1016/j.tet.2020.131632 Achille Antenucci , Margherita Barbero , Stefano Dughera , Giovanni Ghigo
|
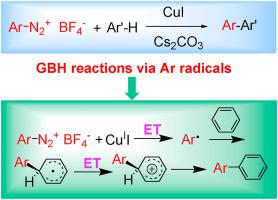
Gomberg-Bachmann-Hey reactions were carried out in the presence of copper as a catalyst and gave rise to biaryls or heterobiaryls in good yields and in mild reaction conditions. A computational study of some key points of the reaction was performed. The results are coherent with the experimental data and confirm some aspects of the mechanism. The reaction free energies for the reduction in benzene by CuI of a set of 40 (hetero)arenediazonium tetrafluoroborates were calculated. Both the experiments and the calculations showed that in the coupling with substituted solvents (toluene, bromobenzene, nitrobenzene and anisole) the binding to the ortho position was always favoured.
中文翻译:

铜催化四氟硼酸芳构氮鎓和邻苯二磺酰亚胺杂芳烃鎓的Gomberg-Bachmann-Hey反应。综合和机械方面
Gomberg-Bachmann-Hey反应在铜作为催化剂的情况下进行,以高收率和温和的反应条件产生联芳基或杂联芳基。对反应的一些关键点进行了计算研究。结果与实验数据一致,并证实了该机理的某些方面。计算了一组40个(杂)芳烯重氮四氟硼酸盐通过CuI还原苯所产生的反应自由能。实验和计算均表明,在与取代溶剂(甲苯,溴苯,硝基苯和苯甲醚)的偶联中,总是倾向于与邻位的结合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号