当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption and Inhibition effect of Tetraaza-tetradentate macrocycle ligand and its Ni (II), Cu (II) Complexes on the corrosion of Cu10Ni alloy in 3.5% NaCl Solutions
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125653 K. Shalabi , Ola. A. El-Gammal , Y.M. Abdallah
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125653 K. Shalabi , Ola. A. El-Gammal , Y.M. Abdallah
|
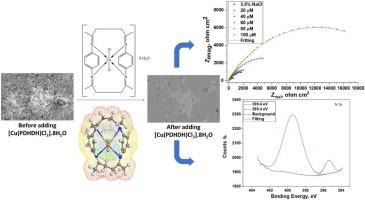
Abstract In an attempt to search for high performance, effective and ecofriendly corrosion inhibitors, the macrocyclic compartmental ligand: (2E)-3,6,10,13-tetramethyl-2,7,9,14-tetraaza-1,8(1,4)dibenzena cyclotetradecaphane-2,6,9,13-tetraene (PDHDH) and its Ni (II), Cu (II) complexes have been synthesized and characterized by elemental and spectroscopic techniques. For corrosion inhibition studies of Cu10Ni alloy, different concentrations of synthesized inhibitors were added to 3.5 % NaCl as corrosive media and surveyed by potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. The surface of alloy samples treated with these inhibitors was analyzed by scanning electron microscope (SEM) and X-ray photoelectron spectroscopy (XPS) to confirm the existence of adsorbed protected film. PDP curves showed that the PDHDH and its Ni (II), Cu (II) complexes behave as mixed type inhibitors. The data demonstrated that the synthesized inhibitors could assist as active inhibitors for Cu10Ni alloy corrosion in 3.5 % NaCl solutions and their protection capacity reached to 95.7 % at 100 μM additive concentration. The synthesized inhibitors obey Freundlich adsorption isotherm, furthermore, the adsorption of these inhibitors on Cu10Ni is physisorption. DFT calculations, Fukui indices, Mulliken atomic charges and molecular electrostatic potential (MEP) mapping were accomplished to support the adsorption features and the corrosion inhibition mechanism of the investigated inhibitors.
中文翻译:

四氮杂-四齿大环配体及其Ni(II)、Cu(II)配合物对Cu10Ni合金在3.5%NaCl溶液中腐蚀的吸附抑制作用
摘要 为了寻找高性能、有效和环保的缓蚀剂,大环室配体:(2E)-3,6,10,13-tetramethyl-2,7,9,14-tetraaza-1,8(1 ,4)dibenzena cyclotetradecaphane-2,6,9,13-tetraene (PDHDH) 及其 Ni (II)、Cu (II) 配合物已被合成并通过元素和光谱技术表征。对于Cu10Ni合金的腐蚀抑制研究,将不同浓度的合成抑制剂加入3.5% NaCl作为腐蚀介质,并通过动电位极化(PDP)、电化学阻抗谱(EIS)和电化学频率调制(EFM)技术进行检测。通过扫描电子显微镜 (SEM) 和 X 射线光电子能谱 (XPS) 分析用这些抑制剂处理的合金样品的表面,以确认吸附保护膜的存在。PDP曲线显示PDHDH及其Ni(II)、Cu(II)配合物表现为混合型抑制剂。数据表明,合成的抑制剂在 3.5% NaCl 溶液中可作为 Cu10Ni 合金腐蚀的活性抑制剂,在 100 μM 添加剂浓度下其保护能力达到 95.7%。合成的抑制剂服从Freundlich吸附等温线,而且这些抑制剂对Cu10Ni的吸附是物理吸附。DFT 计算、福井指数、
更新日期:2021-01-01
中文翻译:

四氮杂-四齿大环配体及其Ni(II)、Cu(II)配合物对Cu10Ni合金在3.5%NaCl溶液中腐蚀的吸附抑制作用
摘要 为了寻找高性能、有效和环保的缓蚀剂,大环室配体:(2E)-3,6,10,13-tetramethyl-2,7,9,14-tetraaza-1,8(1 ,4)dibenzena cyclotetradecaphane-2,6,9,13-tetraene (PDHDH) 及其 Ni (II)、Cu (II) 配合物已被合成并通过元素和光谱技术表征。对于Cu10Ni合金的腐蚀抑制研究,将不同浓度的合成抑制剂加入3.5% NaCl作为腐蚀介质,并通过动电位极化(PDP)、电化学阻抗谱(EIS)和电化学频率调制(EFM)技术进行检测。通过扫描电子显微镜 (SEM) 和 X 射线光电子能谱 (XPS) 分析用这些抑制剂处理的合金样品的表面,以确认吸附保护膜的存在。PDP曲线显示PDHDH及其Ni(II)、Cu(II)配合物表现为混合型抑制剂。数据表明,合成的抑制剂在 3.5% NaCl 溶液中可作为 Cu10Ni 合金腐蚀的活性抑制剂,在 100 μM 添加剂浓度下其保护能力达到 95.7%。合成的抑制剂服从Freundlich吸附等温线,而且这些抑制剂对Cu10Ni的吸附是物理吸附。DFT 计算、福井指数、






































 京公网安备 11010802027423号
京公网安备 11010802027423号