Biomaterials ( IF 12.8 ) Pub Date : 2020-10-05 , DOI: 10.1016/j.biomaterials.2020.120429 Jun Zhang , Jie Yang , Tiantian Zuo , Siyu Ma , Nadira Xokrat , Zongwei Hu , Zhihua Wang , Rui Xu , Yawen Wei , Qi Shen
|
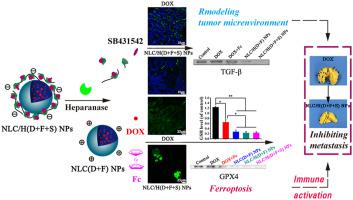
The normal chemotherapy only induces the intracellular apoptosis pathway to promote primary tumor cells death, while not inhibit tumor metastasis. Herein, we proposed a kind of heparanase (HPSE)-driven sequential released nanoparticles, which modified with β-cyclodextrin (β-CD) grafted heparin (NLC/H(D + F + S) NPs) co-loading with doxorubicin (DOX), ferrocene (Fc), and TGF-β receptor inhibitor (SB431542). NLC/H(D + F + S) NPs successfully inhibited breast cancer metastasis by intracellular and extracellular hybrid mechanism. DOX and Fc loaded in NLC/H(D + F + S) NPs effectively enhanced intracellular ROS level to activate ferroptosis pathway, the enhanced ROS also induced the apoptosis pathway and decreased MMP-9 expression to synergize with ferroptosis for tumor therapy. In extracellular site, SB431542 was sequentially released by HPSE-driven, which blocked tumor metastasis by modulating tumor microenvironment, decreasing TAFs activation, and reducing the secretion of TGF-β. In addition, anti-tumor immune response induced by ferroptosis further strengthened the effect of tumor therapy. Finally, under the help of intracellular and extracellular mechanisms launched by NLC/H(D + F + S) NPs, the satisfactory anti-tumor metastasis effect was obtained in the in vivo anti-tumor assays. Therefore, NLC/H(D + F + S) NPs was a novel dosage regimen for breast cancer therapy through intracellular and extracellular mechanisms, in which ferroptosis induced by ROS played an important role.
中文翻译:

乙酰肝素酶驱动的顺序释放纳米颗粒用于乳腺癌治疗中的肥大症和肿瘤微环境调节协同作用
正常化学疗法仅诱导细胞内凋亡途径以促进原发性肿瘤细胞死亡,而不抑制肿瘤转移。在本文中,我们提出了一种由乙酰肝素酶(HPSE)驱动的顺序释放纳米颗粒,该纳米颗粒用与阿霉素(DOX)共负载的β-环糊精(β-CD)接枝肝素(NLC / H(D + F + S)NPs)修饰),二茂铁(Fc)和TGF-β受体抑制剂(SB431542)。NLC / H(D + F + S)NPs通过细胞内和细胞外杂交机制成功抑制了乳腺癌的转移。装载在NLC / H(D + F + S)NPs中的DOX和Fc有效地增强了细胞内ROS的水平以激活肥大化途径,增强的ROS还诱导了细胞凋亡途径并降低了MMP-9的表达以与肥大症协同治疗肿瘤。在细胞外部位,SB431542由HPSE驱动的顺序释放,通过调节肿瘤微环境,减少TAFs活化并减少TGF-β的分泌来阻止肿瘤转移。此外,由肥大症引起的抗肿瘤免疫反应进一步增强了肿瘤治疗的效果。最后,在NLC / H(D + F + S)NPs启动的细胞内和细胞外机制的帮助下,获得了令人满意的抗肿瘤转移效果。体内抗肿瘤试验。因此,NLC / H(D + F + S)NPs是一种通过细胞内和细胞外机制治疗乳腺癌的新型给药方案,其中ROS引起的肥大症起着重要的作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号