当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
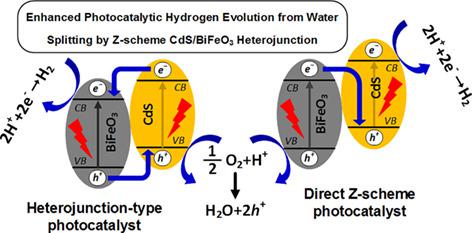
Enhanced photocatalytic hydrogen evolution from water splitting by Z‐scheme CdS/BiFeO3 heterojunction without using sacrificial agent
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-10-02 , DOI: 10.1002/er.5966 Amin Kolivand 1 , Shahram Sharifnia 1
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-10-02 , DOI: 10.1002/er.5966 Amin Kolivand 1 , Shahram Sharifnia 1
Affiliation
|
Cadmium sulfide (CdS) is one of the most famous photocatalyst for water splitting because of relevant band edge position. But it has disadvantage such as recombination of charge carrier and photocorrosion in pure water that sacrificial materials must be used to fix it. On the other hand, the use of other photocatalysts alongside it can greatly eliminate these disadvantages. So, in favor of boosting photocatalytic efficiency, it was coupled with BiFeO3 that is a promising visible light active perovskite structure and has ferroelectric properties. The CdS and BiFeO3 powders were synthesized by precipitation and hydrothermal methods, respectively. The pure samples and heterojunctions were investigated by XRD, FESEM, transmission electron microscopy, EDX, UV‐vis, PL, N2 adsorption/desorption isotherms, photocurrent density measurement, electrochemical impedance spectroscopy and Mott‐Schottky analysis to compare the structure, composition, morphology, and optical property before and after modification. The maximum photocatalytic activity, 600.2 μmol/g/h was obtained by heterojunction containing 50 wt% BiFeO3 (CB‐50) from pure water. Also from the experiments results, CB‐50 showed much higher reusability than pure CdS. The enhanced stability and photocatalytic activity of CB‐50 are mainly attributed to charge separation as a result of Z‐scheme charge transfer mechanism as well as the increased light absorption efficiency. In addition to increasing hydrogen production, the ability to use pure water in a closed system without additional materials is other advantage of this photocatalyst.
中文翻译:

在不使用牺牲剂的情况下,通过Z方案CdS / BiFeO3异质结分解水可增强光催化制氢能力
由于相关的带边缘位置,硫化镉(CdS)是最著名的水分解光催化剂之一。但是它的缺点是,如电荷载体的复合和在纯水中的光腐蚀,必须使用牺牲材料来固定它。另一方面,与之一起使用其他光催化剂可以大大消除这些缺点。因此,为了提高光催化效率,将其与BiFeO 3结合在一起,BiFeO 3是一种很有前途的可见光活性钙钛矿结构,具有铁电性能。用沉淀法和水热法分别合成了CdS和BiFeO 3粉末。通过XRD,FESEM,透射电子显微镜,EDX,UV-vis,PL,N 2对纯样品和异质结进行了研究吸附/解吸等温线,光电流密度测量,电化学阻抗谱和Mott-Schottky分析,以比较改性前后的结构,组成,形态和光学性质。通过含有50 wt%BiFeO 3的异质结获得最大光催化活性600.2μmol/ g / h(CB-50)来自纯净水。同样从实验结果来看,CB-50的可重用性比纯CdS高得多。C方案50的增强的稳定性和光催化活性主要归因于Z方案电荷转移机制以及提高的光吸收效率导致的电荷分离。除了增加氢气产量外,在无附加材料的情况下在密闭系统中使用纯净水的能力是该光催化剂的另一个优势。
更新日期:2020-10-02
中文翻译:

在不使用牺牲剂的情况下,通过Z方案CdS / BiFeO3异质结分解水可增强光催化制氢能力
由于相关的带边缘位置,硫化镉(CdS)是最著名的水分解光催化剂之一。但是它的缺点是,如电荷载体的复合和在纯水中的光腐蚀,必须使用牺牲材料来固定它。另一方面,与之一起使用其他光催化剂可以大大消除这些缺点。因此,为了提高光催化效率,将其与BiFeO 3结合在一起,BiFeO 3是一种很有前途的可见光活性钙钛矿结构,具有铁电性能。用沉淀法和水热法分别合成了CdS和BiFeO 3粉末。通过XRD,FESEM,透射电子显微镜,EDX,UV-vis,PL,N 2对纯样品和异质结进行了研究吸附/解吸等温线,光电流密度测量,电化学阻抗谱和Mott-Schottky分析,以比较改性前后的结构,组成,形态和光学性质。通过含有50 wt%BiFeO 3的异质结获得最大光催化活性600.2μmol/ g / h(CB-50)来自纯净水。同样从实验结果来看,CB-50的可重用性比纯CdS高得多。C方案50的增强的稳定性和光催化活性主要归因于Z方案电荷转移机制以及提高的光吸收效率导致的电荷分离。除了增加氢气产量外,在无附加材料的情况下在密闭系统中使用纯净水的能力是该光催化剂的另一个优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号