当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of chiral urea-derived iodoarenes and their assessment in the enantioselective dearomatizing cyclization of a naphthyl amide
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-03 , DOI: 10.1016/j.tet.2020.131634 M. Umair Tariq , Wesley J. Moran
中文翻译:

手性尿素衍生的碘芳烃的设计与合成及其在萘酰胺对映选择性脱芳香化环化中的评估
更新日期:2020-10-17
Tetrahedron ( IF 2.1 ) Pub Date : 2020-10-03 , DOI: 10.1016/j.tet.2020.131634 M. Umair Tariq , Wesley J. Moran
|
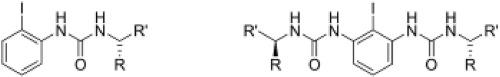
A novel family of urea-derived chiral iodoarenes was designed and synthesized for use in enantioselective iodine(I/III) catalysis. Their preparation required the development of a bidirectional synthetic strategy. These new chiral iodoarenes were assessed as catalysts in the dearomatizing cyclization of a naphthyl amide and provided moderate yields of product in some cases with low enantioselectivities.
中文翻译:

手性尿素衍生的碘芳烃的设计与合成及其在萘酰胺对映选择性脱芳香化环化中的评估
设计并合成了一种新的脲衍生手性碘芳烃家族,用于对映选择性碘(I / III)催化。他们的准备工作需要开发双向合成策略。这些新的手性碘代芳烃被评估为萘酰胺脱芳环化反应中的催化剂,在某些情况下具有低对映选择性的情况下可提供中等产率的产物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号