Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-10-03 , DOI: 10.1016/j.molliq.2020.114473 Jiyaul Haque , Mohammad A. Jafar Mazumder , M.A. Quraishi , S.A. Ali , N.A. Aljeaban
|
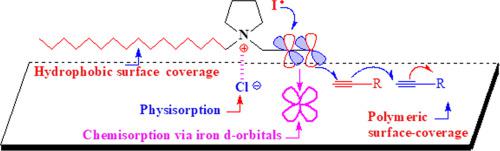
A new series of industrially relevant pyrrolidine based quaternary ammonium salts containing propargyl motif and hydrophobic C-12, C-16 alkyl chains were synthesized in excellent yields and evaluated for mild steel corrosion in 1 M HCl. FT-IR, NMR, and TGA were analyzed to characterize these corrosion inhibitors. The performance of these corrosion inhibitors was examined by gravimetric weight loss, electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP), and theoretical calculations using density functional theory (DFT) methods. The surface morphology of mild steel samples was studied by UV–vis spectroscopy, scanning electron microscopy (SEM) and x-ray photoelectron spectroscopy (XPS). Results revealed that the inhibition capabilities of corrosion inhibitors N, N-dipropargylpyrrolidium bromide (DPPB), N-dodecyl, N-propargylpyrrolidium bromide (DDPPB), N-hexadecyl, N-propargylpyrrolidium bromide (HDPPB) are concentrations, immersion time and temperature-dependent, and found to be very good inhibitors even at a meager concentration of 60.3 μmol L−1 with an efficiency value 92.6, 93.7, and 96.2%, respectively. The inhibition efficiency order of these corrosion inhibitors, as shown by gravimetric and electrochemical studies, is as follows: HDPPB > DDPPB > DPPB. The inhibition efficiencies obtained from gravimetric weight loss, PDP, and EIS measurements are in good agreement. The thermodynamic parameters such as Ea, Kads, ΔG°mic, and ΔG°ads were determined. As compare to ΔG°mic, the more negative ΔG°ads values suggested favorable adsorption over the micellization. The critical micelle concentration (CMC) values of DPPB, DDPPB, and HDPPB were found to be 35.5, 31.8, and 30.0 mmol L−1. At CMC, the percent surface coverage was ≈90%, revealed that the monolayer formation by the inhibitor molecules at the metal/solution interface is almost complete before the concentrations reach their CMC values. Electrochemical studies suggested that corrosion inhibitors DPPB and DDPPB act as a mixed type with predominantly cathodic inhibitors, while HDPPB serves as a cathodic inhibitor. Surface analysis by UV–vis spectroscopy, SEM, and XPS supported the adsorption of inhibitor molecules on the mild steel surface. The DFT analysis is in good agreement with the experimentally obtained results on the molecular structure and adsorption strength.
中文翻译:

含炔丙基和疏水性C-12和C-16烷基链的吡咯烷基季铵盐在水性酸性介质中作为缓蚀剂
合成了一系列新的工业相关的基于吡咯烷的季铵盐,其中含有炔丙基基序和疏水性的C-12,C-16烷基链,并且收率很高,并在1 M HCl中进行了轻度钢腐蚀评估。分析了FT-IR,NMR和TGA以表征这些腐蚀抑制剂。这些腐蚀抑制剂的性能通过重量失重,电化学阻抗谱(EIS),电势极化(PDP)和使用密度泛函理论(DFT)方法的理论计算进行了检验。通过紫外可见光谱,扫描电子显微镜(SEM)和X射线光电子能谱(XPS)研究了低碳钢样品的表面形态。结果表明,缓蚀剂N,N的缓蚀能力-二炔丙基吡咯烷溴化物(DPPB),N-十二烷基,N-炔丙基吡咯烷溴化物(DDPPB),N-十六烷基,N-炔丙基吡咯烷溴化物(HDPPB)是浓度,浸渍时间和温度依赖性,并且即使在60微量浓度为60.3μmolL -1,效率值分别为92.6%,93.7和96.2%。重量分析和电化学研究表明,这些缓蚀剂的缓蚀效率顺序如下:HDPPB> DDPPB> DPPB。从重量失重,PDP和EIS测量获得的抑制效率非常一致。热力学参数,例如E a,K确定了ads,ΔG ° mic和ΔG ° ads。与ΔG ° mic相比,较负的ΔG ° ads值表明在胶束作用下具有有利的吸附作用。发现DPPB,DDPPB和HDPPB的临界胶束浓度(CMC)值为35.5、31.8和30.0 mmol L -1。在CMC处,表面覆盖百分率约为90%,这表明在浓度达到其CMC值之前,抑制剂分子在金属/溶液界面形成的单层几乎完成。电化学研究表明,腐蚀抑制剂DPPB和DDPPB与主要的阴极抑制剂混合使用,而HDPPB作为阴极抑制剂。通过紫外可见光谱,SEM和XPS进行的表面分析支持抑制剂分子在低碳钢表面上的吸附。DFT分析与实验获得的分子结构和吸附强度结果吻合良好。






























 京公网安备 11010802027423号
京公网安备 11010802027423号