当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic Carbene Gold(I) Complexes: Mechanism of the Ligand Scrambling Reaction and Their Oxidation to Gold(III) in Aqueous Solutions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-10-02 , DOI: 10.1021/acs.inorgchem.0c02298 Sina K Goetzfried 1 , Caroline M Gallati 1 , Monika Cziferszky 1 , Radu A Talmazan 2 , Klaus Wurst 2 , Klaus R Liedl 2 , Maren Podewitz 2 , Ronald Gust 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-10-02 , DOI: 10.1021/acs.inorgchem.0c02298 Sina K Goetzfried 1 , Caroline M Gallati 1 , Monika Cziferszky 1 , Radu A Talmazan 2 , Klaus Wurst 2 , Klaus R Liedl 2 , Maren Podewitz 2 , Ronald Gust 1
Affiliation
|
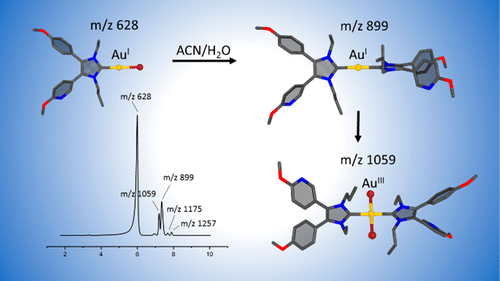
N-Heterocyclic carbene (NHC) gold(I) complexes offer great prospects in medicinal chemistry as antiproliferative, anticancer, and antibacterial agents. However, further development requires a thorough understanding of their reaction behavior in aqueous media. Herein, we report the conversion of the bromido[3-ethyl-4-(4-methoxyphenyl)-5-(2-methoxypyridin-5-yl)-1-propylimidazol-2-ylidene]gold(I) ((NHC)AuIBr, 1) complex in acetonitrile/water mixtures to the bis[3-ethyl-4-(4-methoxyphenyl)-5-(2-methoxypyridin-5-yl)-1-propylimidazol-2-ylidene]gold(I) ([(NHC)2AuI]+, 7), which is subsequently oxidized to the dibromidobis[3-ethyl-4-(4-methoxyphenyl)-5-(2-methoxypyridin-5-yl)-1-propylimidazol-2-ylidene]gold(III) ([(NHC)2AuIIIBr2]+, 9). By combining experimental data from HPLC, NMR, and (LC-)/HR-MS with computational results from DFT calculations, we outline a detailed ligand scrambling reaction mechanism. The key step is the formation of the stacked ((NHC)AuIBr)2 dimer (2) that rearranges to the T-shaped intermediate Br(NHC)2AuI–AuIBr (3). The dissociation of Br– from 3 and recombination lead to (NHC)2AuI–AuIBr2 (5) followed by the separation into [(NHC)2AuI]+ (7) and [AuIBr2]− (8). [AuIBr2]− is not stable in an aqueous environment and degrades in an internal redox reaction to Au0 and Br2. The latter in turn oxidizes 7 to the gold(III) species 9. The reported ligand rearrangement of the (NHC)AuIBr complex differs from that found for related silver(I) analogous. A detailed understanding of this scrambling mechanism is of utmost importance for the interpretation of their biological activity and will help to further optimize them for biomedical and other applications.
中文翻译:

N-杂环卡宾金(I)配合物:配体加扰反应的机理及其在水溶液中氧化成金(III)
N-杂环卡宾 (NHC) 金 (I) 配合物在药物化学中作为抗增殖剂、抗癌剂和抗菌剂具有广阔的前景。然而,进一步的开发需要彻底了解它们在水性介质中的反应行为。在此,我们报告了溴代[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金(I) ((NHC) Au I Br, 1 ) 在乙腈/水混合物中与双[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金络合物( I) ([(NHC) 2 Au I ] + , 7),随后被氧化成二溴双[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金(III)([(NHC ) 2 Au III Br 2 ] + , 9 )。通过将 HPLC、NMR 和 (LC-)/HR-MS 的实验数据与 DFT 计算的计算结果相结合,我们概述了详细的配体加扰反应机制。关键步骤是形成堆叠的 ((NHC)Au I Br) 2二聚体 ( 2 ),该二聚体重新排列为 T 形中间体 Br(NHC) 2 Au I –Au I Br ( 3 )。Br的离解——从3和重组导致 (NHC) 2 Au I –Au I Br 2 ( 5 ) 然后分离成 [(NHC) 2 Au I ] + ( 7 ) 和 [Au I Br 2 ] - ( 8 )。[Au I Br 2 ] -在水性环境中不稳定并且在内部氧化还原反应中降解为Au 0和Br 2。后者反过来将7氧化为金 (III) 物种9. 报道的 (NHC)Au I Br 配合物的配体重排不同于相关银 (I) 类似物的配体重排。详细了解这种加扰机制对于解释它们的生物活性至关重要,并将有助于进一步优化它们以用于生物医学和其他应用。
更新日期:2020-10-21
中文翻译:

N-杂环卡宾金(I)配合物:配体加扰反应的机理及其在水溶液中氧化成金(III)
N-杂环卡宾 (NHC) 金 (I) 配合物在药物化学中作为抗增殖剂、抗癌剂和抗菌剂具有广阔的前景。然而,进一步的开发需要彻底了解它们在水性介质中的反应行为。在此,我们报告了溴代[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金(I) ((NHC) Au I Br, 1 ) 在乙腈/水混合物中与双[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金络合物( I) ([(NHC) 2 Au I ] + , 7),随后被氧化成二溴双[3-乙基-4-(4-甲氧基苯基)-5-(2-甲氧基吡啶-5-基)-1-丙基咪唑-2-亚基]金(III)([(NHC ) 2 Au III Br 2 ] + , 9 )。通过将 HPLC、NMR 和 (LC-)/HR-MS 的实验数据与 DFT 计算的计算结果相结合,我们概述了详细的配体加扰反应机制。关键步骤是形成堆叠的 ((NHC)Au I Br) 2二聚体 ( 2 ),该二聚体重新排列为 T 形中间体 Br(NHC) 2 Au I –Au I Br ( 3 )。Br的离解——从3和重组导致 (NHC) 2 Au I –Au I Br 2 ( 5 ) 然后分离成 [(NHC) 2 Au I ] + ( 7 ) 和 [Au I Br 2 ] - ( 8 )。[Au I Br 2 ] -在水性环境中不稳定并且在内部氧化还原反应中降解为Au 0和Br 2。后者反过来将7氧化为金 (III) 物种9. 报道的 (NHC)Au I Br 配合物的配体重排不同于相关银 (I) 类似物的配体重排。详细了解这种加扰机制对于解释它们的生物活性至关重要,并将有助于进一步优化它们以用于生物医学和其他应用。












































 京公网安备 11010802027423号
京公网安备 11010802027423号