Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.scitotenv.2020.142618 Haoyu Luo , Yijie Wang , Xiaoqing Wen , Shuailong Cheng , Jie Li , Qintie Lin
|
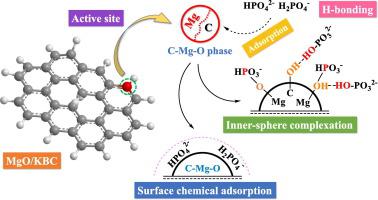
The affinity of biochar (BC) adsorbing phosphate was weak, while generation of magnesium oxide (MgO)-BC nanocomposites that transformed the crystal structures of BC would change the adsorption processes in improving the phosphate adsorption. Hereon, four different crystal structure of absorbents were selected to illustrate why the crystal structures and surface properties of absorbents were of great importance for the phosphate adsorption. The results showed that MgO/KBC with higher combination degree between MgO and KBC could change the normal crystal structure (MgO/KBC1, MgO phase (dominant)) to C-Mg-O phase (dominant). Therefore, MgO/KBC could achieve highest adsorption rate (k2, 8.059 g mg-1 min-1) and qm (maximal adsorption capacity, 121.950 mg g-1) for phosphate adsorption among absorbents, and even it had high anti-interference capacity for anions and natural organic matter (NOM). The mechanisms of MgO/KBC for phosphate adsorption were hydrogen-bond interaction, inner-sphere complexation and surface chemical adsorption. While, adsorption of phosphate on MgO/KBC1 was mainly controlled by inner-sphere complexation (Mg-O-PO3H2-, Mg-O-PO3H2- species). In addition, the adsorbability of MgO/KBC for phosphate could be restored after recalcination, which further proved that an efficient nanocomposite, calcinated from waste biomass (fallen leaves), was proposed to control eutrophication.
中文翻译:

MgO-生物炭纳米复合材料晶体结构对增强磷酸盐吸附的关键作用
生物炭(BC)吸附磷酸盐的亲和力较弱,而生成转化镁晶体结构的氧化镁(MgO)-BC纳米复合材料将改变吸附过程,从而改善磷酸盐的吸附。在此,选择了四种不同的吸收剂晶体结构来说明为什么吸收剂的晶体结构和表面性质对磷酸盐的吸收非常重要。结果表明,MgO与KBC结合度较高的MgO / KBC可以将正常晶体结构(MgO / KBC1,MgO相(占优势))转变为C-Mg-O相(占优势)。因此,MgO的/ KBC可以达到最高吸附率(K 2,8.059克毫克-1分钟-1)和q米吸收剂中最大的吸附量(最大吸附量121.950 mg g -1),甚至对阴离子和天然有机物(NOM)也具有很高的抗干扰能力。MgO / KBC吸附磷酸盐的机理是氢键相互作用,内球络合和表面化学吸附。同时,磷酸对MgO / KBC1的吸附主要是由内球络合控制氧化镁(MgO-PO 3 ħ 2-,氧化镁-PO 3 H ^ 2 -种)。此外,重新煅烧后可以恢复MgO / KBC对磷酸盐的吸附性,这进一步证明,提出了一种由废生物质(落叶)煅烧的有效纳米复合材料,以控制富营养化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号