当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the mechanism of catalytic synthesis of dimethyldichlorosilane by AlCl3/MIL‐53(Al)@γ‐Al2O3
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-09-29 , DOI: 10.1002/aoc.6030 Wenyuan Xu 1 , Yan Wang 1 , Suying Li 1 , Yongbing Cheng 1 , Zanru Guo 1 , Lin Hu 1 , Mengyin Liao 1 , Jiaxi Peng 1 , Xi Chen 1 , Shaoming Yang 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2020-09-29 , DOI: 10.1002/aoc.6030 Wenyuan Xu 1 , Yan Wang 1 , Suying Li 1 , Yongbing Cheng 1 , Zanru Guo 1 , Lin Hu 1 , Mengyin Liao 1 , Jiaxi Peng 1 , Xi Chen 1 , Shaoming Yang 1
Affiliation
|
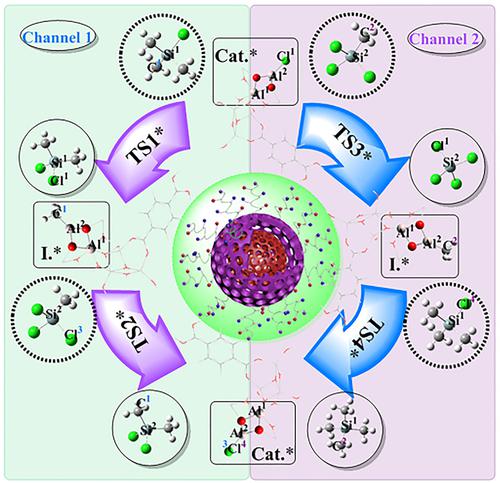
Dimethyldichlorosilane, one of the most consumed organosilicon monomers in the industry, can be prepared in a highly efficient and environmentally friendly synthesis method of disproportionating methylchlorosilanes. However, the internal mechanism of the reaction remains unclear. In this paper, the mechanism catalyzed by AlCl3/MIL‐53(Al) and AlCl3/MIL‐53(Al)@γ‐Al2O3 catalysts was calculated at B3LYP/6‐311++G(3df, 2pd) level by using the density functional theory (DFT). The results showed that although the two catalysts had similar active structures, the catalytic effects were significantly different. The Lewis acid center on the surface of γ‐Al2O3 in the core‐shell catalyst is complementary to the classic Lewis acid AlCl3 through the spatial superposition effect, which greatly improves the Lewis acid catalytic activity of AlCl3/MIL‐53(Al)@γ‐Al2O3.
中文翻译:

AlCl3 / MIL‐53(Al)@ γ‐Al2O3催化合成二甲基二氯硅烷的机理研究
二甲基二氯硅烷是工业上最消耗的有机硅单体之一,可以通过高效且环保的甲基氯硅烷歧化合成方法来制备。但是,反应的内部机理仍然不清楚。本文以B3LYP / 6‐311 ++ G(3df,2pd)计算了AlCl 3 / MIL‐53(Al)和AlCl 3 / MIL‐53(Al)@ γ‐Al 2 O 3催化剂的催化机理。密度泛函理论(DFT)。结果表明,尽管两种催化剂具有相似的活性结构,但催化效果却明显不同。的表面上的路易斯酸中心的γ-Al 2 ö 3核-壳型催化剂中的路易斯酸通过空间叠加效应与经典的路易斯酸AlCl 3互补,从而大大提高了AlCl 3 / MIL‐53(Al)@ γ‐Al 2 O 3的路易斯酸催化活性。
更新日期:2020-09-29
中文翻译:

AlCl3 / MIL‐53(Al)@ γ‐Al2O3催化合成二甲基二氯硅烷的机理研究
二甲基二氯硅烷是工业上最消耗的有机硅单体之一,可以通过高效且环保的甲基氯硅烷歧化合成方法来制备。但是,反应的内部机理仍然不清楚。本文以B3LYP / 6‐311 ++ G(3df,2pd)计算了AlCl 3 / MIL‐53(Al)和AlCl 3 / MIL‐53(Al)@ γ‐Al 2 O 3催化剂的催化机理。密度泛函理论(DFT)。结果表明,尽管两种催化剂具有相似的活性结构,但催化效果却明显不同。的表面上的路易斯酸中心的γ-Al 2 ö 3核-壳型催化剂中的路易斯酸通过空间叠加效应与经典的路易斯酸AlCl 3互补,从而大大提高了AlCl 3 / MIL‐53(Al)@ γ‐Al 2 O 3的路易斯酸催化活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号