当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flat crown ethers with planar tetracoordinate carbon atoms
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-09-28 , DOI: 10.1002/qua.26479 Krishnan Thirumoorthy 1 , Venkatesan S. Thimmakondu 2
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-09-28 , DOI: 10.1002/qua.26479 Krishnan Thirumoorthy 1 , Venkatesan S. Thimmakondu 2
Affiliation
|
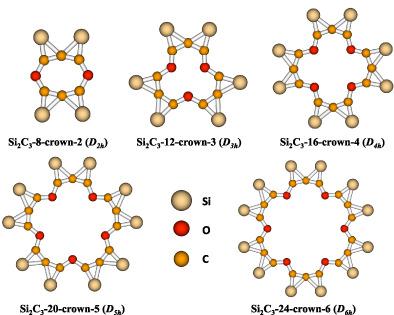
Novel flat crown ether molecules have been characterized in silico using density functional theory. Monomer units of Si2C3 with a planar tetracoordinate carbon (ptC) atom have been used as building blocks. Alkali (Li+, Na+, K+, Rb+, and Cs+) and alkaline‐earth (Be2+, Mg2+, Ca2+, Sr2+, and Ba2+) metal ions, and uranyl (UO ) ion selective complexes have also been theoretically identified. The high symmetry and higher structural rigidity of the host molecules may likely impart higher selectivity in chelation. The potential energy surface of the parent elemental composition, Si2C3H2, has been investigated using coupled‐cluster (CC) approximation. The molecule with a ptC atom within the latter is a low‐lying isomer lying 12.41 kcal mol−1 above the global minimum at the CCSD(T)/cc‐pVTZ level. The crown ether molecules identified here could theoretically be considered the derivatives of the ptC atom isomer. Theoretical binding energies (ΔE; 0 K) and thermally corrected Gibbs free energies (ΔG; 298.15 K) for crown ether molecules have been computed to gauge their binding affinities.
) ion selective complexes have also been theoretically identified. The high symmetry and higher structural rigidity of the host molecules may likely impart higher selectivity in chelation. The potential energy surface of the parent elemental composition, Si2C3H2, has been investigated using coupled‐cluster (CC) approximation. The molecule with a ptC atom within the latter is a low‐lying isomer lying 12.41 kcal mol−1 above the global minimum at the CCSD(T)/cc‐pVTZ level. The crown ether molecules identified here could theoretically be considered the derivatives of the ptC atom isomer. Theoretical binding energies (ΔE; 0 K) and thermally corrected Gibbs free energies (ΔG; 298.15 K) for crown ether molecules have been computed to gauge their binding affinities.
中文翻译:

平面四配位碳原子的平面冠醚
新型扁平冠醚分子已使用密度泛函理论在计算机上进行了表征。具有平面四配位碳(ptC)原子的Si 2 C 3的单体单元已用作构建基块。碱金属离子(Li +,Na +,K +,Rb +和Cs +)和碱土金属(Be 2 +,Mg 2 +,Ca 2 +,Sr 2+和Ba 2+)和铀酰( O 离子选择性配合物也已在理论上得到鉴定。主体分子的高对称性和较高的结构刚性可能会赋予螯合较高的选择性。母体元素组成Si 2 C 3 H 2的势能面已使用耦合簇(CC)近似进行了研究。后者中具有ptC原子的分子是一个低层异构体,位于CCSD(T)/ cc-pVTZ水平上,比全球最低值高12.41 kcal mol -1。从理论上讲,这里鉴定出的冠醚分子可以认为是ptC原子异构体的衍生物。理论结合能(Δ é ; 0 K)和热校正吉布斯自由能(Δ ģ; 已计算出冠醚分子的298.15 K)来衡量其结合亲和力。
离子选择性配合物也已在理论上得到鉴定。主体分子的高对称性和较高的结构刚性可能会赋予螯合较高的选择性。母体元素组成Si 2 C 3 H 2的势能面已使用耦合簇(CC)近似进行了研究。后者中具有ptC原子的分子是一个低层异构体,位于CCSD(T)/ cc-pVTZ水平上,比全球最低值高12.41 kcal mol -1。从理论上讲,这里鉴定出的冠醚分子可以认为是ptC原子异构体的衍生物。理论结合能(Δ é ; 0 K)和热校正吉布斯自由能(Δ ģ; 已计算出冠醚分子的298.15 K)来衡量其结合亲和力。
更新日期:2020-09-28
 ) ion selective complexes have also been theoretically identified. The high symmetry and higher structural rigidity of the host molecules may likely impart higher selectivity in chelation. The potential energy surface of the parent elemental composition, Si2C3H2, has been investigated using coupled‐cluster (CC) approximation. The molecule with a ptC atom within the latter is a low‐lying isomer lying 12.41 kcal mol−1 above the global minimum at the CCSD(T)/cc‐pVTZ level. The crown ether molecules identified here could theoretically be considered the derivatives of the ptC atom isomer. Theoretical binding energies (ΔE; 0 K) and thermally corrected Gibbs free energies (ΔG; 298.15 K) for crown ether molecules have been computed to gauge their binding affinities.
) ion selective complexes have also been theoretically identified. The high symmetry and higher structural rigidity of the host molecules may likely impart higher selectivity in chelation. The potential energy surface of the parent elemental composition, Si2C3H2, has been investigated using coupled‐cluster (CC) approximation. The molecule with a ptC atom within the latter is a low‐lying isomer lying 12.41 kcal mol−1 above the global minimum at the CCSD(T)/cc‐pVTZ level. The crown ether molecules identified here could theoretically be considered the derivatives of the ptC atom isomer. Theoretical binding energies (ΔE; 0 K) and thermally corrected Gibbs free energies (ΔG; 298.15 K) for crown ether molecules have been computed to gauge their binding affinities.
中文翻译:

平面四配位碳原子的平面冠醚
新型扁平冠醚分子已使用密度泛函理论在计算机上进行了表征。具有平面四配位碳(ptC)原子的Si 2 C 3的单体单元已用作构建基块。碱金属离子(Li +,Na +,K +,Rb +和Cs +)和碱土金属(Be 2 +,Mg 2 +,Ca 2 +,Sr 2+和Ba 2+)和铀酰( O
 离子选择性配合物也已在理论上得到鉴定。主体分子的高对称性和较高的结构刚性可能会赋予螯合较高的选择性。母体元素组成Si 2 C 3 H 2的势能面已使用耦合簇(CC)近似进行了研究。后者中具有ptC原子的分子是一个低层异构体,位于CCSD(T)/ cc-pVTZ水平上,比全球最低值高12.41 kcal mol -1。从理论上讲,这里鉴定出的冠醚分子可以认为是ptC原子异构体的衍生物。理论结合能(Δ é ; 0 K)和热校正吉布斯自由能(Δ ģ; 已计算出冠醚分子的298.15 K)来衡量其结合亲和力。
离子选择性配合物也已在理论上得到鉴定。主体分子的高对称性和较高的结构刚性可能会赋予螯合较高的选择性。母体元素组成Si 2 C 3 H 2的势能面已使用耦合簇(CC)近似进行了研究。后者中具有ptC原子的分子是一个低层异构体,位于CCSD(T)/ cc-pVTZ水平上,比全球最低值高12.41 kcal mol -1。从理论上讲,这里鉴定出的冠醚分子可以认为是ptC原子异构体的衍生物。理论结合能(Δ é ; 0 K)和热校正吉布斯自由能(Δ ģ; 已计算出冠醚分子的298.15 K)来衡量其结合亲和力。














































 京公网安备 11010802027423号
京公网安备 11010802027423号