当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Mediated α-Amino Alkylation of Azomethine Imines: An Approach to N-(β-Aminoalkyl)pyrazolidinones
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.orglett.0c02821 Bianca T. Matsuo 1 , José Tiago M. Correia 1 , Márcio W. Paixão 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.orglett.0c02821 Bianca T. Matsuo 1 , José Tiago M. Correia 1 , Márcio W. Paixão 1
Affiliation
|
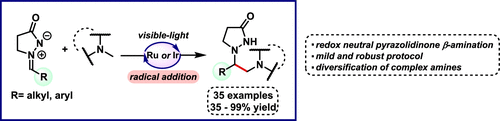
Herein, a mild and robust photocatalytic protocol for the combination of amino and pyrazolidinone functionalities through a radical α-amino alkylation of azomethine iminium ions is demonstrated. This method presents a high functional group tolerance providing direct access to a large family of N-(β-aminoalkyl)pyrazolidinones in good to excellent yields, including the late-stage incorporation of the pyrazolidinone moiety to pharmaceutical ingredients. We propose a plausible scenario for the C–C bond-forming step which involves radical addition followed by a spin-center-shift event.
中文翻译:

可见光介导的甲亚胺亚胺的α-氨基烷基化:N-(β-氨基烷基)吡唑烷酮的一种方法
在本文中,展示了一种温和且坚固的光催化方案,用于通过偶氮甲碱亚胺离子的自由基α-氨基烷基化来结合氨基和吡唑烷酮官能团。该方法具有很高的官能团耐受性,可以以良好至极佳的收率直接进入大家族的N-(β-氨基烷基)吡唑烷酮,包括将吡唑烷酮部分后期掺入药物成分中。我们为C–C键形成步骤提出了一种可行的方案,该方案涉及自由基加成,然后发生自旋中心移位事件。
更新日期:2020-10-17
中文翻译:

可见光介导的甲亚胺亚胺的α-氨基烷基化:N-(β-氨基烷基)吡唑烷酮的一种方法
在本文中,展示了一种温和且坚固的光催化方案,用于通过偶氮甲碱亚胺离子的自由基α-氨基烷基化来结合氨基和吡唑烷酮官能团。该方法具有很高的官能团耐受性,可以以良好至极佳的收率直接进入大家族的N-(β-氨基烷基)吡唑烷酮,包括将吡唑烷酮部分后期掺入药物成分中。我们为C–C键形成步骤提出了一种可行的方案,该方案涉及自由基加成,然后发生自旋中心移位事件。































 京公网安备 11010802027423号
京公网安备 11010802027423号