当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Novel Anthraquinone‐Containing Poly(Triphenylamine) Derivative: Preparation and Electrochemical Performance as Cathode for Lithium‐Ion Batteries
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-09-27 , DOI: 10.1002/celc.202001084 chang su 1 , Bing Han 2 , Jinpeng Ma 2 , Lihuan Xu 3
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-09-27 , DOI: 10.1002/celc.202001084 chang su 1 , Bing Han 2 , Jinpeng Ma 2 , Lihuan Xu 3
Affiliation
|
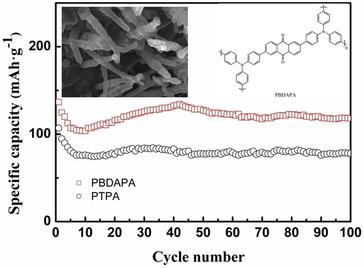
Organic and polymeric materials are excellent candidates for next generation advanced electrode materials. Therein, 2,6‐Bis(4‐(diphenylamino)phenyl)‐9,10‐anthracenedione (BDAPA) functional monomer was synthesized through Suzuki coupling reaction, and a novel anthraquinone‐containing poly(triphemylamine) polymer (PBDAPA) was then prepared by the simple oxidative polymerization. The obtained novel functional polymer presented a unique urchin‐like morphology with outgrowth of hollow tubular spiny, which possesses the improved specific surface area of ∼129.6 m2 g−1 and the small average mesopore diameter of 1.78 nm. As cathode material, the obtained PBDAPA compared to polytriphenylamine (PTPA), demonstrated two obvious discharge plateaus, corresponding to the double charge‐discharge characteristics from p‐type triphenylamine and n‐type anthraquinone segments in the polymer, respectively. Also, PBDAPA exhibited an improved specific capacity of 132.7 mAh g−1 and an enhanced rate capability with the discharge specific capacities of 140.6, 124.3, 107.5 and 97.1 mAh g−1 at the discharge rates of 20, 50, 100 and 200 mA g−1, respectively. The introduction of anthraquinone unit in polytriphenylamine as well as the resulted open pore morphology for PBDAPA was responsible to the improved electrochemical performances, which makes it a potential strategy for the design and preparation of high performance organic lithium‐ion batteries.
中文翻译:

新型蒽醌类聚三苯胺衍生物:锂离子电池的制备及电化学性能
有机和聚合物材料是下一代高级电极材料的极佳候选材料。其中,通过Suzuki偶联反应合成了2,6-双(4-(二苯氨基)苯基)-9,10-蒽二酮(BDAPA)功能单体,然后制备了一种新型的含蒽醌的聚三苯胺聚合物(PBDAPA)通过简单的氧化聚合。所获得的新型功能聚合物表现出独特的海胆状形态,并长出空心管状的刺状棘齿,具有比表面积约129.6 m 2 g -1的改善。小平均中孔直径为1.78 nm。作为正极材料,与聚三苯胺(PTPA)相比,所得PBDAPA表现出两个明显的放电平稳期,分别对应于聚合物中p型三苯胺和n型蒽醌链段的双重充放电特性。同样,PBDAPA在20、50、100和200 mA g的放电速率下表现出比重提高的132.7 mAh g -1和比速率的提高,放电比容量为140.6、124.3、107.5和97.1 mAh g -1 -1, 分别。在聚三苯胺中引入蒽醌单元以及PBDAPA产生的开孔形态有助于改善电化学性能,这使其成为设计和制备高性能有机锂离子电池的潜在策略。
更新日期:2020-10-15
中文翻译:

新型蒽醌类聚三苯胺衍生物:锂离子电池的制备及电化学性能
有机和聚合物材料是下一代高级电极材料的极佳候选材料。其中,通过Suzuki偶联反应合成了2,6-双(4-(二苯氨基)苯基)-9,10-蒽二酮(BDAPA)功能单体,然后制备了一种新型的含蒽醌的聚三苯胺聚合物(PBDAPA)通过简单的氧化聚合。所获得的新型功能聚合物表现出独特的海胆状形态,并长出空心管状的刺状棘齿,具有比表面积约129.6 m 2 g -1的改善。小平均中孔直径为1.78 nm。作为正极材料,与聚三苯胺(PTPA)相比,所得PBDAPA表现出两个明显的放电平稳期,分别对应于聚合物中p型三苯胺和n型蒽醌链段的双重充放电特性。同样,PBDAPA在20、50、100和200 mA g的放电速率下表现出比重提高的132.7 mAh g -1和比速率的提高,放电比容量为140.6、124.3、107.5和97.1 mAh g -1 -1, 分别。在聚三苯胺中引入蒽醌单元以及PBDAPA产生的开孔形态有助于改善电化学性能,这使其成为设计和制备高性能有机锂离子电池的潜在策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号